ClearPoint in the News
Visit us regularly to stay up-to-date on the latest mentions of ClearPoint Neuro and our innovative technology from news outlets and media from around the globe
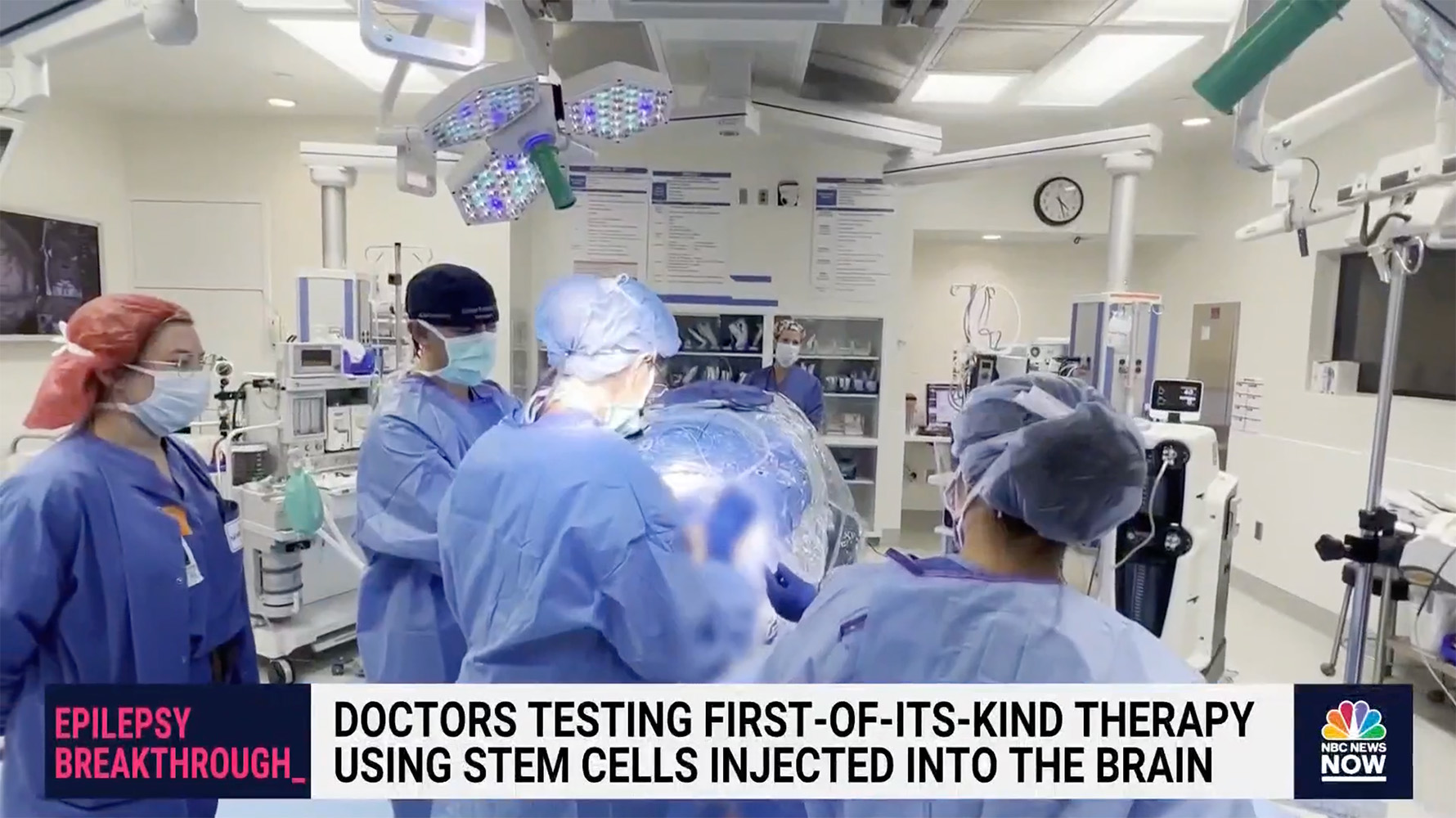
Doctors Test Experimental Treatment to Combat Seizures in Epilepsy Patients
Source: Online News Segment from NBC News NOW
September 25th, 2023
Epilepsy Breakthrough: Doctors testing first-of-its-kind therapy using stem cells injected into the brain with investigational procedure.
Doctors are testing a promising experimental treatment using stem cells to combat seizures in patients with epilepsy. NBC News’ Dr. Akshay Syal spoke with a clinical trial patient about her experience.
The ClearPoint team would like to extend our congratulations to Dr. Sharona Ben-Haim and the entire neurosurgery team at UC San Diego on successfully delivering Neurona Therapeutics' groundbreaking investigational regenerative cell therapy for Drug Resistant Mesial Temporal Lobe Epilepsy! ClearPoint is proud to have supported the investigational procedure with our MRI-guided navigation platform and SmartFlow Cannula delivery device.
Simone’s Story with a Happy Ending: An Example of Collaboration Between People, Science, & Institutions
Source: Online Article from OMAR: Osservatorio Malattie Rare - Deficit di AADC
June 27th, 2023
The child, suffering from an extremely rare disease known as AADC deficiency, successfully received gene therapy at the Policlinico Umberto I in Rome.
Rome - In March, the story of Simone, a Sicilian child suffering from a very rare disease, AADC deficiency, made headlines after his parents, Sabrina and Sebastiano, had written a heartfelt letter to the government, and in particular to Prime Minister Giorgia Meloni and the Undersecretary of State with Delegated Responsibility for Rare Diseases the Hon. Marcello Gemmato, asking for help so that their son could receive the treatment he urgently needed and that other children had already received in France and Germany, as Osservatorio Malattie Rare [Rare Diseases Observatory] also reported.
Simone's trials and tribulations started at 6 months of age, when the baby, during one of the classic pediatric checkups, could not hold his head up, his muscles were weak, and he held his arms wide open and his fists closed. This led to two weeks of hospitalization at the San Marco Hospital in Catania until arriving, in January 2021, thanks to the advice of a neuro-psychiatrist who was monitoring him in Sicily, at the IRCCS Fondazione Stella Maris in Pisa, where the little boy was soon diagnosed and given treatment. His care and treatment were then extended to Policlinico Umberto I in Rome, a designated center of excellence for gene therapy of this ultra-rare disease. Simone was the only one of 16 Italian patients with AADC deficiency eligible to receive the innovative gene therapy capable of changing the natural course of his disease, eladocagene exuparvovec, an innovative orphan drug developed by PTC Therapeutics. Although the therapy is approved at the European level, it had not yet finished its access process in Italy. Because of this, the family's wait had already stretched for a year, a huge amount of time in the face of a degenerative disease.
There are approximately 200 cases of AADC deficiency worldwide and it is severely underdiagnosed. “It is a rare inherited neurometabolic disorder, with autosomal recessive transmission, caused by biallelic mutations in the DDC gene,” explained Professor Roberta Battini, Head of UOS Departmental Clinic of Neurological Disorders and Rare Diseases, IRCCS Fondazione Stella Maris in Calambrone (PI), the specialist who diagnosed Simone with the disease and has been monitoring him for two years now. “Clinical manifestations are generally evident in the first months of life and the most frequent include hypotonia, i.e., decreased muscle tone; hypokinesia, i.e., reduced or slow voluntary body movements; oculogyric seizures and autonomic nervous system dysfunction. In most cases, AADC deficiency presents in a severe form, but there are some cases of some patients with a milder form of the disease.”
The family’s appeal was promptly taken up by the institutions: Prime Minister Meloni and Undersecretary Gemmato personally telephoned the family several times, and in parallel initiated a close dialogue with AIFA, Policlinico Umberto I and PTC Therapeutics. A quick and efficient organization, so much so that on May 22, only a month after from AIFA's go-ahead, the long-awaited moment of intervention arrived.
Eladocagene exuparvovec is the first advanced therapy in the world to involve administration directly into the brain and is indicated for patients with AADC deficiency, aged 18 months or older, with a severe phenotype, as in the case of little Simone. In fact, the child, despite being 3 years old, still cannot speak or walk. Without treatment, his condition would have further degenerated as the natural history of this very rare and progressive genetic disease teaches us, while today the expectations are radically different: in fact, Simone is expected to be able to recover several lost growth stages.
“In Europe there are already three specialized centers for this type of innovative procedure: Montpellier, Paris and Heidelberg,” confirmed Riccardo Ena, Executive Director, Head of Spain, Italy & Portugal, PTC Therapeutics. “So today we are pleased to have contributed to a decisive and substantial change in Simone's life, and our goal, as PTC Therapeutics, is precisely to focus on the combination of research and therapeutic opportunity, to be at the service of patients.”
A Success Story in Which Scientific Research and Dialogue Have Played a Key Role:
Simone’s story is not just a story with a happy ending. It is the triumph of scientific research, which, even to people with very rare diseases, has once again managed to provide treatment which can change the natural history of a disease. And it is at the same time the tangible sign of the success that can be achieved through dialogue, collaboration and teamwork that, in this case (already declared by other European countries at international benchmarks) has involved the government, especially Prime Minister Giorgia Meloni and the Undersecretary of State with Delegation for Rare Diseases Hon. Marcello Gemmato, AIFA, with the direct intervention of President Giorgio Palù and Directors Anna Rosa Marra and Pierluigi Russo, Riccardo Ena of PTC Therapeutics, and the team of (medical, health and administrative) specialists at Policlinico Umberto I - La Sapienza, who put in place the most impressive clinical safety measures so that everything could go smoothly.
The first obstacle, the regulatory one, was overcome by resorting to the early access procedure established by Act 326/2003, Fund 5%, which allows, in extremely urgent circumstances, access to specific funding for treatment approved in Europe by the EMA even before the completion of the Italian authorization processes. Having taken this first step, thanks to the fundamental support of AIFA, which is responsible for this authorization, it was necessary to get the Polyclinic to quickly handle all the administrative and organizational aspects, which happened in record time, and the health aspects, given the complexity and absolute novelty of this procedure. The organization lasted almost a month, during which time the MRI room, inaugurated last year, was adapted to a neurosurgery room, numerous rehearsals of the procedure were carried out, checking everything down to the smallest detail, and all the pre-operative visits were carried out, until the go-ahead came for the procedure.
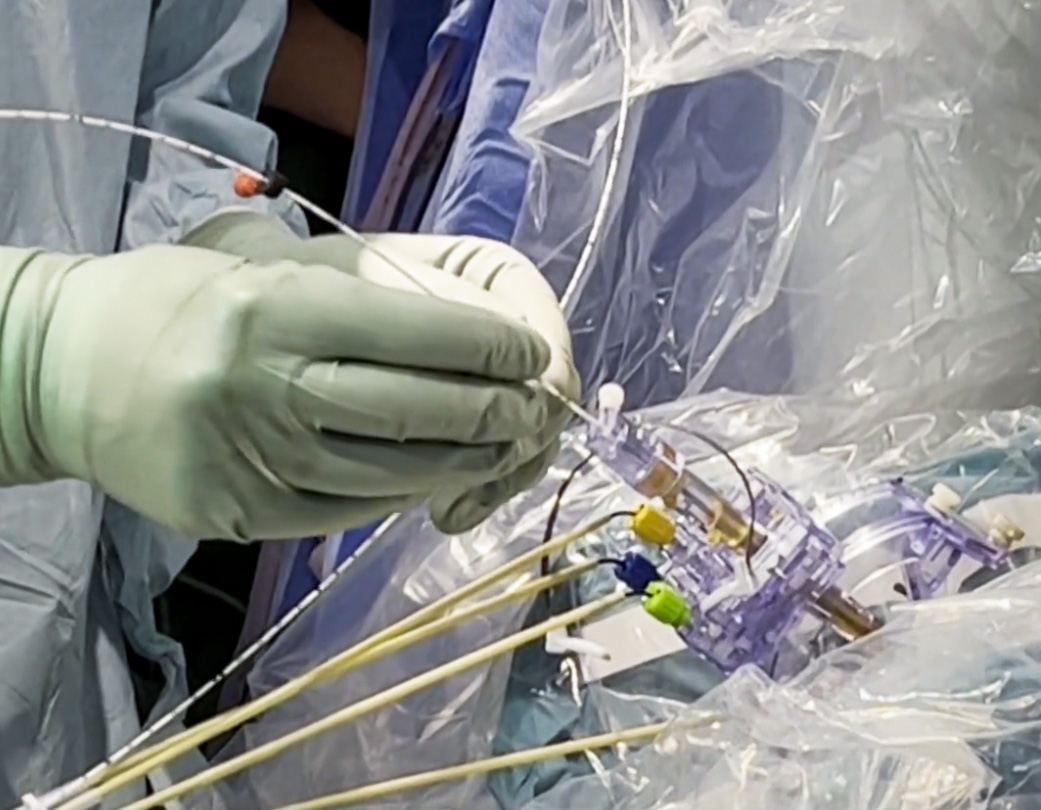
It was an operation that lasted a full eight hours that involved the use, also for the first time in Italy and in the world, of state-of-the-art instrumentation, capable of bringing, through specific cannulas, the therapy to the target points of the brain (i.e. to certain areas of the putamen) with absolute and necessary precision. “Prior to the marketing authorization of eladocagene exuparvovec by the EMA last year, there were no approved treatments to help these children,” said Jeremy Stigall, Executive Vice President and General Manager of Biologics and Drug Delivery at ClearPoint Neuro, the company that developed this instrumentation. “Our European team is pleased to have supported the first patient in Italy to receive eladocagene exuparvovec, infused through our combined SmartFlow Cannula solution, guided by ClearPoint Navigation in the MRI”.
The entire, highly complex operation was performed by a team of operators and specialists who worked intensively to ensure efficacy and zero risk for the patient: the surgery was performed by Prof. Antonio Santoro, Director of Neurosurgery, and Dr. Luca D'Angelo, Neurosurgeon, Department of Neuroscience and Mental Health, AOU Policlinico Umberto I in Rome, supported by Prof. Francesco Pisani, Director-in-Charge, UOC of Child Neuropsychiatry, AOU Policlinico Umberto I in Rome.
We would therefore like to thank the following people: Giorgia Meloni, Marcello Gemmato, Giorgio Palù, Anna Rosa Marra, Pierluigi Russo, Roberta Battini, Francesco Pisani, Luca D’Angelo, Antonio Santoro, Fabrizio d’Alba, Matteo Galletta, Roberto Poscia, Jacopo Loy, Enrica Proli, Luigi Fiorito, Giacomo Polito, Fabrizio Minuto, Paolo Gazzaniga, Matthew Rabon, George Protopapas, Federica Pappone, Nicola De Blasiis, Carmela Imperiale, Maria Vittoria Pesce, Patrizia Fedeli, Francesco Pugliese, Vittoria Santillo, Patrizia Pantano, Marta Iacobucci, Valeria Panebianco, Luigi Della Volpe, Alessandro Di Pietro, Rosalba Floccari, Paola Papoff, Carlo Cefali, Cristina Cannizzaro, Daniele Licata, Laura De Vito, Maria Augurusa, Raffaella Marchettini, Massimiliano Coluzzi, Laura Franceschetti, Vincenzo Falco, Sara Patisso, Laura Crippa, PTC Therapeutics supply chain TEAM.
By clicking HERE, you can watch OMaR's video interview with Sebastiano, Simone's dad, who relates the entire story.
The procedure for eladocagene exuparvovec therapy was outlined at a press conference organized by the Azienda Ospedaliero-Universitaria Policlinico Umberto I and Sapienza University of Rome in collaboration with Osservatorio Malattie Rare and with a non-conditional contribution from PTC Therapeutics.
De-Risking Cell And Gene Therapy Clinical Trials
Source: Online Article from Cell & Gene - Guest Column
May 25th, 2023
By Russell Lonser, M.D., Ohio State University College of Medicine, Department of Neurological Surgery; Professor and Chair, Department of Neurological Surgery; and Director, Ohio State University Gene Therapy Institute.
Historical Overview
The direct delivery of gene therapy drugs to the brain is a nascent, promising, and rapidly evolving field. My interest was first piqued in 1996 - 98 when I was a fellow at the National Institutes of Health (NIH) with Dr. Ed Oldfield, who directed the first clinical trial of gene therapy within the central nervous system (CNS) during the mid 1990s. In 1994, citing poor penetration of drugs infused into brain parenchyma, Dr. Oldfield was one of the pioneers in a new drug delivery technique called convection-enhanced delivery (CED), which utilizes a continuous hydrostatic pressure gradient to distribute macromolecules into the interstitial spaces of the brain parenchyma.1 Since then, I have been fascinated with the intricate properties and morphologies of directed delivery into the brain, brainstem, and spinal cord. I returned to the NIH as faculty in 2001 and we conducted multiple gene therapy trials in tumors and evolving over the last 10 to 15 years, into neurodegenerative and other neurologic disorders. I became Chairman of the Ohio State University Wexner Medical Center in 2012 and earlier this year, launched the Gene Therapy Institute at Ohio State along with my colleague Dr. Krystof Bankiewicz.
From a chronological perspective, there have been many iterative steps that have brought us to where we are today; finally seeing successes in early-stage gene therapy trials via direct delivery to the brain. First, there were biologic and physical properties issues we had to understand. Then, from the early '90s into the mid-2000s, there were device-related challenges that were very different for tumors versus neurologic disorders. Furthermore, the evolution from preclinical to clinical and understanding where to place the drugs inside the brain, interpreting the properties and applying them to humans was extremely complex.
Limitations in Direct Delivery Led to Early Trial Failures
Local delivery of therapeutic agents into the brain has many advantages; however, the inability to predict, visualize, and confirm the infusion into the intended target has been the major hurdle. I attribute five major reasons for the early failures in direct delivery gene therapy trials. The biggest challenge initially, was knowing where we were infusing and where the drug was going in the brain.
The second challenge was the technologies used to perform the procedures. For example, going back even 20 years, we were using ventricular catheters, which were not designed for infusion. In addition, there was no way to easily reposition or move the cannulas. So, available technology influenced our approach, which I would consider the third limitation. Early on, we approached the brain from the top, due to the available equipment at the time. We've since changed approaches to different areas within the brain including anterior, posterior or lateral.
The fourth limitation, and still an issue today, concerns the experience of the institute or specialty center. We see this evolution with every new kind of surgical technology. Early phase trials in brain tumors, for example, had varying degrees of success (or not) based on the experience of the center and its clinicians / surgeons. Inconsistency in delivery across staff, sites and diseases resulted in skewed results. Like any complex surgery, its experience, know-how, and currency, that results in reproducible results.
And last, drug development plays a key role. We’ve seen drugs that had excellent results in small animal models, but those results did not carry over into humans.
Methods and Technologies to Increase the Efficacy of Direct Drug Delivery
Advancements in imaging, planning, and delivery platforms have enabled improved targeting, accurate catheter placement, better drug distribution, and real-time visualization of infusions, resulting in reduced risks and enhanced success in clinical trials. Furthermore, a redesign of the cannula has resulted in more reliable, efficient, and increased distribution of agents.2 Collectively, these enhancements have opened a whole new era that we didn't have in the '90s.
New image guided systems provide real-time visualization of the infusion using a gadolinium-based co-infusate, enabling instantaneous alteration of infusion parameters, including flow rate, catheter repositioning or termination when necessary.3
The ClearPoint Neuro Navigation platform brings the frameless stereotactic MR compatible system together with the SmartFlow cannulas for targeted placement, making us highly accurate in the operating room. Because the platform is MR compatible, it allows us to view our cannula infusions in real-time with a high degree of accuracy and reliability. The cannulas were specifically designed to do gene therapy, which resolves previous problems such as clumping or adherence. And they allow us the flexibility to reposition them during the infusions.
Techniques have also evolved as a result of enhanced technologies. I’ve spent the bulk of my research, focusing on ways to image directed delivery utilizing an interventional MRI (iMRI) system for catheter placement and visualization of infusion. We applied CED of therapeutic agents with iMRI across several different clinical trials in neuro-oncology and movement disorders. We learned we could match surrogate tracers with the drug to see with a great degree of precision, where the therapeutic is going.
That informs us about the properties in various disease types of delivery. It also enhances our accuracy because we can see leak back along the cannula track and can slow the rate. As well it enhances safety because we'll only infuse the areas necessary and then stop the flow.
Another thing we've learned is ‘infuse as you go,’ which is moving the cannula in real time so that we can paint the structure that we're infusing because there are very few or no purely spherical structures in the brain. The ability to see, in real time, where the drug is infusing and continuously correct to get the optimal infusion volume is very advantageous, because gene therapy dosing in the brain is all about the amount of the structure you're covering. Obviously, your vector is important, but once you’re tracking, the goal is to completely fill the structure you want to treat, dissimilar to giving a drug intravenously or through other methods.
Imaging also informs us about efficacy. If we treat the targeted area and there is no impact, that is probably not an efficacious drug. On the other hand, as we're learning, if we treat larger areas, we have a dose effect based on the volume treated. This has been tremendously valuable in our understanding of how to better deliver and how to define efficacy.
Continued developments and accumulated experience with the techniques and technologies of drug formulations, CED platforms, and intraoperative MR systems is making local therapeutic delivery into the brain more accurate, efficient, effective, and safer.
Collaboration to Speed Success
As clinicians, we work closely with both pharma companies and industry. At the Gene Therapy Institute, we have 10 active clinical trials in gene therapy. My advice to pharma companies is select trialists that are currently conducting cell and gene therapy trials and involve them early in the process. We are often approached when the pharma company has already set up their IND enabling studies in a way that we wouldn't in a clinical trial, so then you may lose one or two years reconfiguring your trial. I have the greatest respect for the scientists – they are the disease experts. And we are the treatment experts. Close collaboration between the scientists and the surgeons during this stage of the trial is really critical. And this is a shift in thinking from 10 or 15 years ago, when preclinical trials were viewed as ‘do it the way it would make sense from a small animal model’ versus looking at the much larger human brain size related issues you must overcome for success. We also actively partner with industry in the development of the hardware, software and targeting that we use. Again, this helps to streamline the process and results in products designed specifically for our needs.
Enhanced Success Rate in Phase I / Phase II
The benefits of direct delivery of certain therapies are immense for both the patients and the pharma industry. From an efficacy standpoint, there's significant early data in phase one and two that look exceptionally promising across several disorders. A recent example is MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons in children. Phase one results were striking in terms of health improvement, and the study demonstrated midbrain gene delivery in children with AADC deficiency is feasible and safe, and leads to clinical improvements in symptoms and motor function. The trial continues in a more extended phase one.4
There’s a lot of optimism now that some trials have shown early success. For diseases where we understand the circuitry, we can use the directed delivery and place concentrated levels of drug into targeted areas and therapeutically manipulate those circuits. Increasingly, through targeted delivery, we are also reducing the potential for off-target effects, which commonly occur with therapeutics delivered systemically.
Planning for the Future
In the United States, we have approximately 60 centers that have intra-operative MRI, and only a few that do directed gene therapy into the brain. I think as these agents advance in the clinic and become approved for therapy, that will create an opening for this technology. And technology advancement begets training, fellowships, and certifications just like any new surgical or interventional therapy. It’s not just placing the catheter to a specific spot, it’s learning how to shape those infusions, learning how to manage different anatomic borders between gray and white, between the brain and the ventricular system because those create different infusion profiles or delivery profiles, than simply placing it into an area with homogeneous consistency.
The Gene Therapy Institute at Ohio State holds one of the largest first-in-human clinical trial portfolios in nervous system gene therapy. Throughout the year, we invite doctors and pharma to come and watch our procedures. Several have begun creating their own kind of training regimen, whereby a surgeon who wants to initiate these infusions will come here, view several cases, and then we'll go and watch them do several cases at their home institution. And prior, their institution will be reviewed for infrastructure, workflow and the like, because in Phase I and Phase II trials, you need that consistency to show what your therapeutic is doing. You can’t have a portion of your patients being treated inconsistently – it’s impossible to win that back in a trial.
From an economic standpoint, we need to find ways to further streamline the time in the operating room, the time it takes an intraop MR, and then ultimately, we need new technologies that won't require us to use the intraop MR imaging to facilitate real-time understanding of where the drug is going. That may be predictive software, and it may be a whole host of things that will allow us to do that.
Predictions for Gene Therapy
I think the future of gene therapy is super exciting. I predict a few things will occur. For example, as we garner greater biologic insight into the underlying causes of a number of these degenerative and other disorders, we'll have better targeted gene therapies that can narrow in on very specific targets. This, in turn, will allow us to disrupt and therapeutically improve disordered circuits within the brain. And there is some evidence that we can keep this behind the blood-brain barrier, which would reduce potential for a negative immune impact. Less certain, is whether we could potentially redose a patient. These are things we're studying today.
Secondly, and in parallel, hardware and software technologies will continue to evolve at a rapid pace, which will also improve our understanding of delivery and how to do it efficiently and impactfully. Lastly, expertise will increase and become more prevalent.
And when I discuss the future, it is not 10 years away, rather we will see these changes, in significant ways, in the next 2 to 5 years. These advancements are creating an electrifying future for gene therapy. FDA predictions for registration submissions of gene therapeutics are expected to rise exponentially over the next several years and that's across all gene therapeutics. And it looks like we are within reach of achieving FDA’s prediction that it will be approving 10 to 20 cell and gene therapy products per year by 2025.5 Now that is very motivating for pharma, industry, and patients!
About the Author
I am a neurological surgeon who specializes in the resection of brain, spinal cord, pituitary and brainstem tumors. I also serve as professor and chair of the Department of Neurological Surgery at The Ohio State University College of Medicine. I hold the Dardinger Family Chair in Neurosurgical Oncology. Previously, I was chief of the Surgical Neurology Branch at the National Institutes of Health. My research includes nervous system drug delivery, gene therapy and tumor biology. I have authored more than 300 publications. I received the Tumor Young Investigator Award and the Mahaley Clinical Research Award from the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Tumor Section. Additionally, I was the AANS/CNS Tumor Section Bittner Lecturer and the American Academy of Neurological Surgery Edward H. Oldfield Lecturer.
I have served as treasurer and president of the CNS, and I am currently the Secretary of the American Board of Neurological Surgeons. I was the head of the research subcommittee in Head, Neck and Spine Injury Committee for the National Football League and have served on the editorial boards of NEUROSURGERY, World Neurosurgery, Journal of Neurosurgery, PLoS One and Science Reports. I am also consulting editor for Neurosurgery Clinics of North America and have edited three neurosurgical textbooks.
References
1 Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080.
2 Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J Neurosci Methods. 2010;187(1):46–51. doi: 10.1016/j.jneu-meth.2009.12.008.
3 Richardson RM, Kells AP, Martin AJ, et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotact Funct Neurosurg. 2011;89(3):141–151. doi: 10.1159/000323544.
4 Pearson, T.S., Gupta, N., San Sebastian, W. et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat Commun 12, 4251 (2021). https://doi.org/10.1038/s41467-021-24524-8
The patient was enrolled in a Glioblastoma clinical trial using the ClearPoint Prism™ Neuro Laser Therapy System at Skåne University Hospital in Lund, Sweden. He was the third patient in Europe to undergo a minimally invasive brain tumor ablation procedure with ClearPoint Prism under MRI-guidance.
With a new laser method, which is now being tested in Lund, Petar Vicevic's hard-to-reach brain tumor was surgically removed. And already two days after the operation he was able to go home again.
Just over two years ago, he was operated on for the first time with traditional surgery, where the skull bone was opened. And in January this year, Peter Vicevic in Lomma suffered a cancer recurrence when a new brain tumor was discovered.
"I had prepared for the cancer to come back, but you get completely crushed", says Petar Vicevic.
But this time the opportunity to participate in a new study in Lund appeared. Petar became the third patient in Europe to remove the tumor with a new laser equipment. Through a small hole in the skull, the entire operation was carried out inside a magnetic camera.
"The opportunity did not exist six months ago", he says.
Two days later, Petar was able to leave the hospital.
The Safety & Feasibility of MR-Guided Laser Thermal Ablation of Brain Lesions Study, sponsored by Clinical Laserthermia Systems AB, is a single-center, single-arm, prospective clinical trial evaluating the safety & feasibility of using the minimally invasive MR-guided laser therapy system to ablate tumors in up to 5 Glioblastoma patients.
Source: Online Article from +Mass Device
August 24th, 2022
The Utah Array platform [Image from Blackrock Neurotech/ClearPoint Neuro].
ClearPoint Neuro
ClearPoint Neuro in July 2021 entered into an agreement with none other than Blackrock Neurotech to develop an automated surgical solution for implanting BCIs into patients with neurological disorders — including from paralysis, ALS, blindness and hearing loss.
Solana Beach, Calif.–based ClearPoint Neuro at the time that the partnership aims to leverage its platform and software with Blackrock Neurotech’s Utah Array to deliver a clinical solution for surgeons that is more streamlined and effective than other BCI implantation surgeries performed to date.
Earlier this year, ClearPoint announced a collaboration with Durham, North Carolina–based Higgs Boson Health to bring to market a patient-facing digital application based on the ManageMySurgery platform. The application will specialize in drug delivery to the brain and spine, as well as BCI technology.
Source: Online Article from University of Minnesota Department of Neurosurgery - News & Events
August 19th, 2022
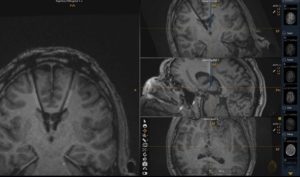
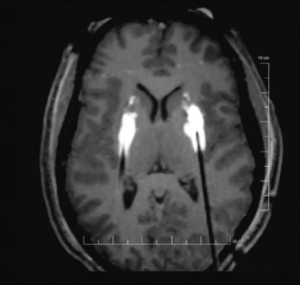
Final electrode location (right side) from the intraoperative MRI images taken during the procedure.
On June 28, 2022, functional neurosurgeon Michael C. Park, MD, PhD, performed the first deep brain stimulation (DBS) procedure in Minnesota that used the MRI-guided ClearPoint™ Neuro system. He was treating an epilepsy patient and found that the technology provided distinct advantages.
Using DBS to treat intractable epilepsy was approved by the FDA in 2018. The procedure delivers electrical stimulation to the anterior nucleus of the thalamus (ANT), a small brain structure involved in the spread of an initially localized seizure. “The ANT really can’t be mapped using electrophysiology to determine where you are when placing the electrodes,” said Park. “It’s a very small area in the brain – less than a centimeter in diameter.”
Tricky procedure
Prior to using ClearPoint Neuro, these procedures could be tricky, according to Park (pictured here). “You would have to look at an image, pick a target, and place the electrode,” he explained. Unlike DBS procedures to treat movement disorders, the patient cannot be awake to help the neurosurgeon understand if the electrodes are positioned accurately. “If an epilepsy patient is awake during surgery, you might trigger a seizure,” said Park.
Pre-op, intra-op, and post-op CT scans and fluoroscopic X-ray images are also required during the typical DBS procedure for epilepsy. “That kind of imaging gives you indirect confirmation of where you are,” said Park. “The procedure takes a long time and is complicated to navigate, and in the end, you really don’t know where your electrode is with any degree of confidence.”
Making it more precise
With the ClearPoint Neuro system, Park used IMRIS intraoperative MRI, another advanced technology available to neurosurgery at University of Minnesota Medical Center, which makes the process much more precise and eventually, will reduce time in the OR. He can also use the ClearPoint Neuro system to plan his approach. “You can put in a cannula or a sheath to get to the target, insert a slender probe known as a stylet, which shows up on the MRI, confirm that’s where you want to go, and then place the electrode,” said Park. “You know where you are in real-time with no guessing. That’s ClearPoint Neuro’s advantage. It’s very accurate with little room for error relative to being off target.”
Department Head Clark C. Chen, MD, PhD, was using the ClearPoint Neuro system for brain tumor surgeries and recommended its use to Park. To learn how to use the system, Park went to Mayo Jacksonville in Florida to observe neurosurgeon Sanjeet Grewal, MD, who uses ClearPoint Neuro with intraoperative MRI on a regular basis.
Excited by the possibilities
As more people with epilepsy turn to DBS to manage their symptoms, Park is excited about the possibilities that ClearPoint Neuro gives him to improve patient outcomes. He and his surgical team have completed two surgeries using the technology and both patients are doing well. Additional procedures are being planned.
“Based on the first two cases, I really like the precision, accuracy, and real-time feedback from the intraoperative MRI,” said Park. “The technology isn’t too difficult to learn how to use, either. And as we become more proficient, I believe it will help us cut down surgery time.”
Source: Online Article from Diagnostic Imaging®
August 11th, 2022
The Maestro Brain Model reportedly provides automated identification, quantification and labeling of brain structures on magnetic resonance imaging (MRI).
Offering the promise of more efficient reporting of findings from magnetic resonance imaging (MRI) of the brain, the Maestro Brain Model has garnered 510(k) clearance from the Food and Drug Administration (FDA), according to the software’s manufacturer ClearPoint Neuro.
Combining deformable surfaces with active shape models and machine learning, the Maestro Brain Model can provide neuroradiologists with automated labeling as well as shape and volumetric quantification of brain structures on MRI scans.
ClearPoint Neuro said the anatomical segment analysis tool emerged from research examining volumetric and shape abnormalities caused by mild traumatic brain injuries. Noting cross-validation of the technology with more than 1,000 MRI scans, the company noted the Maestro Brain Model offers accurate and highly reproducible results.
The company says future applications of the Maestro Brain Model may facilitate targeted treatment of brain injuries.
“Our plan is to quantify drug delivery using intraoperative imaging and simulate patient-specific infusions in targeted brain regions,” explained Lyubomir Zagorchev, a vice-president of Clinical Science and Applications at ClearPoint Neuro. “The unique shape representation in (the Maestro Brain Model) will provide reproducible lead placement for deep brain stimulation and micro electrode recording. Surface meshes of segmented anatomical regions will define safety zones and optimal trajectories for patient-specific laser ablations.”
Clinical Laserthermia Systems (CLS) AB receives 510(k) clearance for the ClearPoint Prism™ Neuro Laser Therapy System. ClearPoint Neuro has exclusive global rights to commercialize the CLS magnetic resonance (MR) guided laser interstitial thermal therapy (MRgLITT) system for neuro applications.
ClearPoint Neuro Inc. said Clinical Laserthermia Systems AB (CLS), snared a 510(k) from the FDA for a laser interstitial therapy system that will be marketed in the U.S. as part of the ClearPoint Prism Neuro system. This product package adds to a growing ClearPoint footprint in the neurological disorders space, adding to an inventory that already consists of targeted drug delivery and deep brain stimulation systems.
Solana Beach, Calif.-based ClearPoint Neuro, which until roughly two years ago went by the name of MRI Interventions, nudged its way into the limelight in this end of the neurosurgery space nearly a decade ago when it reported on developments associated with the first iteration of its namesake system. The company had won a 510(k) for the system three years earlier, but was carefully expanding its market with eight centers in the U.S. and two in Europe at that point in time.
David Franco, general manager of ClearPoint’s laser business operations, told BioWorld, that the current operating configuration includes the laser system by Lund, Sweden-based CLS and thermometry by Pessac, France-based Image Guided Therapy SA, in addition to ClearPoint’s surgical management system, which includes a dedicated laptop computer, a console and surgical instruments. Franco confirmed that the 510(k)for the CLS component is agnostic for tissue type and location, but that it generally calls for necrotizing and coagulating soft tissue through interstitial irradiation or thermal therapy under MRI guidance.
This system will typically be used for brain cancers and other intracranial tumors, but Franco said epilepsy is one potential addition to the indication for use for the ClearPoint. The big advantage in this class of surgical interventions is that these systems typically require the creation of a surgical access point of only 3-4 millimeters in many instances, whereas craniotomy was required in times gone by for epilepsy treatments.
This configuration allows the surgeon to conduct the procedure within the MRI torus without breaking the sterile barrier even though the gantry must slide out and back in from time to time. Among the other companies working in this space are Monteris Medical Inc., and Medtronic plc (for its Visualase unit), but Franco said ClearPoint has a compelling story to tell customers. For one, the system’s fiberglass tip does not heat up like the polymerase tips used by the competition, which eases workflow concerns on the part of hospital administrators and neurosurgeons alike.
The second competitive advantage is that ClearPoint can offer all a neurosurgeon’s needs from just one vendor, something Franco said was on the wish list for many clinicians and hospital administrators. Beyond that, this combination of products allows the surgeon to confirm the trajectory of the laser insertion device while the patient is inside the MRI and before the need to commit to placing the catheter. "The Thermoguide software loaded into the ClearPoint navigation platform is highly accurate for voxel size and refresh rates as well", Franco said, adding that the company has 2,000 cases in evidence to back the company’s claims about the ClearPoint platform.
Franco said the ClearPoint Prism™ moniker will be used across the globe, adding that the company’s next geographic play is the European Union. Pursuant to a CE mark, the company has obtained regulatory approval for a clinical study that teams the company up with Lund University in Lund, Sweden, but after that, ClearPoint will be “looking opportunistically” at nations in Asia and South America.
From early on, there were other projects in other spaces that ClearPoint management saw as attractive, but the novel technology behind the ClearPoint platform, which was invented via a collaboration between company scientists and their counterparts at Johns Hopkins, seemed to suggest the neurosurgery space was the best application. “That was understood early on and became the focus shortly after the company was founded", Franco said, adding that this focus was solidified with the name change.
The company’s share prices have oscillated quite a bit since February of 2021, when they reached roughly $29, but share prices (NASDAQ: CLPT) are struggling to stay above $10 in late afternoon Sept. 26 trading.Franco said CEO Joseph Burnett is clear about the operational plan. “Our company continues to grow on multiple levels,” Franco said, such as revenues and case volumes. Franco said a number of factors that are affecting med-tech share prices generally are plaguing ClearPoint as well, but he added, “If you look at our stock compared to comparable baskets of stocks, we’re actually doing quite well.”
Franco said ClearPoint has a strong cash position, and thanks to this latest approval and other sources of good news, “we’re going to continue to see share price growth".
Source: Online Article from Dartmouth Health - News & Stories
March 14th, 2022
Joshua P. Aronson, MD, a neurosurgeon at Dartmouth-Hitchcock Medical Center (DHMC), is pictured here during the procedure to implant a neurostimulator device targeting four areas of the brain into Jessica Sargent, an epilepsy patient.
More than 3 million people in the United States live with epilepsy. Of those individuals, about one in six cannot control their seizures with medications or surgery alone. Uncontrolled seizures can lead to an unpredictable, diminished quality of life—without the ability to predict when a seizure might interrupt the day, cause loss of consciousness, or a medical emergency.
Dartmouth-Hitchcock Medical Center’s (DHMC) Department of Neurology and Section of Neurosurgery have partnered with the Yale School of Medicine and the Mayo Clinic to pioneer the Stimulation of the Thalamus for Arousal Restoral in Temporal lobe epilepsy (START) clinical trial—the first to implant neurostimulator devices targeting four specific areas of the brain. Following implantation, participants chosen for this study receive an Epilepsy Personal Assistant Device (EPAD) tablet computer to track seizures and epilepsy medications, as well as an Automatic Responsiveness Testing in Epilepsy (ARTiE) smartwatch, which asks the participant questions during a seizure to see if they are can respond, and determine if the neurostimulator is successfully preventing loss of consciousness.
On February 21, 2022, Jessica Sargent, a 34-year-old mother of an 11-year-old daughter and 7-year-old son from Barrington, NH, became the first START clinical trial participant nationwide to have a neurostimulator device implanted in her brain at DHMC.
“Most of the time, I have no idea when a seizure is going to happen,” Sargent said, adding that she blacked out from a seizure while giving birth to her daughter. “I seem to miss so much of my life.”
Sargent’s surgery was performed at DHMC by neurosurgeon and trial site investigator Joshua P. Aronson, MD, in collaboration with Barbara C. Jobst, MD, chair of the Dartmouth-Hitchcock Department of Neurology and Neurocritical Care and principal site investigator of START; and Hal Blumenfeld, MD, PhD, director of the Yale Clinical Neuroscience Imaging Center, a renowned scientist in consciousness research, and the leader of the national START study. The surgery involved precise placement of neurostimulator electrodes in four brain areas, relying on advanced neurosurgical targeting performed in the Center for Surgical Innovation at DHMC.
Sargent developed epilepsy at 19 and, prior to her surgery, was experiencing as many as three seizures every month. She was unable to work, and her condition caused anxiety in her daily life—not knowing if she could do something as simple as taking her children outside to play without experiencing a seizure.
When Sargent was told that the START trial could give her a chance to remain coherent through seizures, she knew this could be the solution she’s spent 15 years searching for. Three weeks out from her surgery and recovering, she has a positive outlook for her life going forward—and the potential START could have for others with epilepsy.
“When they said I’d be able to help other people, it clicked because I don’t want anyone else to have to go through this,” Sargent said. “It’s been so long since I’ve been having seizures and now with the neurostimulator device, I’m very hopeful.”
The advent of neuromodulation technology in the last decade has been a major step forward in how epilepsy is treated—changing the way the brain networks from inside via an implanted neurostimulator device—but further improvement was needed. Previously, implanted devices would target just two regions of the brain to reduce, but not eliminate seizures. Most patients continue to experience seizures with loss of consciousness. This novel procedure is set to change that.
“The intention of this groundbreaking device is not only to stop seizures from happening but to prevent debilitating seizure symptoms, such as loss of consciousness,” Aronson said. “Ideally, if the device can’t stop the seizure, it will transform how the person experiences it and keep them awake and aware. They may not even know they’re having a seizure because of this new neurostimulator.”
“While everyday tasks like cooking dinner, walking our children to the bus stop, or merely not having to worry that you might lose consciousness without warning, are not causes for concern for most people, for people living with epilepsy, these are real fears they live with constantly,” Jobst said. “The START study offers a chance at normalcy, something most of us take for granted, for epilepsy patients. We’re tremendously grateful that Jessica stepped up and volunteered to be the first to have this procedure, paving the way for others and making her just as critical to START’s success as its investigators. As northern New England’s only Level-4 epilepsy center, DHMC is uniquely poised to be a leader in novel epilepsy treatments, and we look forward to continuing to work with Yale and Mayo Clinic to make this life-changing procedure a reality for epilepsy patients everywhere.”
A Closer Look: Accessing the Deep Brain
Source: Print Article from National Geographic - The Brain: June, 2022 Edition
June 10th, 2022
A pioneering surgical technique treats a tough form of epilepsy while also giving a rare close-up of the insula, a structure crucial to consciousness and treacherous to access.
Camille LaRock’s seizures looked nothing like the ones people see in movies. She stayed fully conscious and could move well enough to play soccer in the midst of a seizure. The only outside sign was a twitch in her eye, a slight droop in her face, and an uncontrollable moan emanating from her lips. The seizures began in middle school, and disrupted her life throughout her teens, sometimes multiple times a day. She was on so much anti-seizure medication at one point that she had to lean against the wall to walk between classes. And yet, even with all that medication, the seizures didn’t stop.
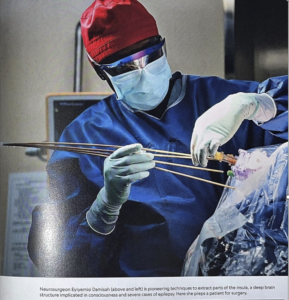
“I felt like I wasn’t really living,” the 19-year-old says. With nothing to lose, she signed up for an experimental surgery at the hands if Eyiyemisi Damisah, a neurosurgeon at the Yale School of Medicine. When LaRock was 17, Damisah robotically reached into her brain to remove a tiny sliver of the insula, an area not commonly seen or accessed.
Exploring the Insula
The insula is “like an island,” Damisah says. It helps maintain homeostasis, arousal, and consciousness while hidden beneath critical layers of cortex that orchestrate our big life experiences—memory, language, movement, decision-making. From the body, blood vessels course into the brain, getting smaller, but denser, the deeper they go. For a neurosurgeon entering someone’s brain, damaging the tiny vessels surrounding the insula would be catastrophic. The insula is “kind of a treacherous region actually to sample,” says Damisah, among the first to forge ahead.
The procedure has two steps. First, while a patient is awake, Damisah robotically places electrodes deep inside the brain. Partly she’s waiting for a seizure to occur naturally so she can watch how it moves through the patient’s brain. But being able to access this area of the brain while a person is alive and awake is so novel in itself that the electrodes also help her learn more about what the elusive insula actually does.
Damisah says many of her patients’ seizures make them feel crazy—their bodies suddenly get hot, they experience untethered distressing emotions, or in LaRock’s case a low moan turned into a full-blown scream as she got older. “The insula, the way I look at it, is like a compact brain itself,” she says. “All the functions you have in the big cortex, you actually have a lot of those in the insula.” So unusual seizure symptoms may actually be a sign of epilepsy originating inside the insula. After the electrode diagnosis and placement are complete, the patient will return for the second surgery, during which they’re unconscious, and Damisah removes a small sample of the insula as a seizure-busting method.
She believes this tricky little structure to be the origin of many kinds of seizures that spread throughout the brain and remain unresponsive to other surgeries and medications. So far, post-surgery, LaRock has been seizure free.
Source: News Segment from TVN24 Poland News Television
February 21st, 2022
TVN24 interview with Polish Neurosurgeon Dr. Mirosław Ząbek - how ClearPoint technology enables partner uniQure to administer potential one-time gene-therapy approach for the treatment of Huntington's Disease in their EU open-label Phase Ib/II clinical trial of AMT-130.
iMRI-Guided Gene Therapy and Drug Delivery for CNS Disorders
Source: Online Publication from BioPharm International
January 19th, 2021
Leveraging real-time MRI guidance for intracranial gene therapy administration potentially improves efficacy and outcomes.
Introduction
While pharmaceutical companies spend billions of dollars developing groundbreaking gene therapies and drugs to address complex diseases of the central nervous system (CNS) (1), the method of delivery is often relegated to an afterthought. With interest and investment in this area continuing to grow at a rapid rate, it is increasingly important to maximize the therapeutic efficacy of both the drug and the delivery method.
Neurodegenerative disorders and brain cancers are two of the most devastating human diseases, and the world’s aging population continues to increase the frequency of these disorders, making rapid and successful drug development more important than ever. Yet, CNS drug development faces extraordinary hurdles and typically takes much longer than non-CNS therapies. Gene therapy clinical trials for CNS diseases are particularly challenging and protracted due to complexities of the brain, potential side effects, the need for direct and often targeted infusion, and delivery complications. Ultimately, the impact of these life changing CNS drugs is being diminished by the “physics of infusion”. Improved delivery methods must be considered and adopted early in the drug development process, or we risk negating their potential therapeutic benefits (2).
The following discusses the current limitations of traditional drug delivery into the brain, reviews recent advancements and benefits of real time interventional MRI (iMRI)-guided gene therapies, describes some ongoing clinical trials, and provides advice for pharmaceutical companies on how to proceed with intracranial therapies and delivery.
Current limitations of CNS drug delivery
It is well known that local delivery of therapeutic agents into the central nervous system offers advantages, including circumventing the blood-brain barrier, the potential for minimizing systemic toxicity, and the ability to achieve higher concentrations of agents at the target site (3). Intracranial delivery has been performed using traditional neurosurgical techniques and tools, including stereotactic frames and standard cannulas or catheters.
These methods lack the ability to confirm accurate catheter placement. Another major challenge has been the inability to visualize the drug delivery in real-time—thus making it impossible to evaluate the accuracy and coverage of drug delivery within target stuctures (4).
This traditional delivery method has become known as “blind” infusion; surgeons would infuse the prescribed amount of the drug to the target area but could not ascertain whether the drug covered the target as intended. In addition, traditional blind delivery with a simple hand injection using a syringe does not allow for infusing at a consistent flow rate, making measurement of delivery rates inaccurate and variable across procedures (4).
Gene therapy trials using traditional intracranial delivery methods began almost 15 years ago, but early results showed limited initial success (4). For example, the PRECISE trial, a large-scale Phase III trial delivering interleukin 13 (IL13)-pseudomonas exotoxin for recurrent glioblastoma, failed to show clinical benefit of survival. Post hoc analysis of the trial patients revealed that less than half of the implanted catheters were in the optimal position—in fact, only 68% of catheters were positioned in accordance with the specified protocol. Additionally, the investigators described “a fundamental lack of infusion distribution physics” as a major reason for efficacy failures. In the years since, similar outcomes have been noted in other trials (3).
Traditional surgical image guidance in the operating room is not real time, but rather dependent on co-registration to a previously-acquired MRI. In addition to the errors that co-registration can introduce with regards to device placement, conventional neuro-navigation platforms offer no means of visualizing infusions in real-time. Without live image guidance, a surgeon has no way to ensure accurate catheter placement and monitor the pattern of distribution of the infusions within the intended target (3).
Initially, surgeons believed that an infusion cannula could be placed into the center of a brain target and the injected fluid would behave in a predictable fashion, similar to an injection of fluid into a beaker of homogenous gelatinous material. The assumption was that the infusion would grow as a uniform spherical ball, increasing in size proportional to an increase in infusion volume. Surgeons now know this is not the case. For example, infusions in the putamen can result in spread of infusate outside the putamen along perivascular spaces, reflux along the inserted cannula, and, to a lesser extent, leakage into the vacated catheter tract upon its removal. Utilizing real-time imaging has enabled surgeons to observe these delivery complications and test new techniques and devices to optimize results.
The advent of real time iMRI-guided infusion has enabled larger volumes of delivered therapeutics and tactics, such as varying infusion rates and adjusting cannula depth/position to maximize coverage of the target area—and to minimize the degree of vector spread away from the intended target. This has significant implications for the success of clinical trials, as evidence suggests that adequate coverage of the target area may have a dramatic impact on patient outcomes (3).
Recent advancements
For pharmaceutical and biologics companies, the inability to predict, visualize, and confirm infusate coverage of target regions has proven to be a major hurdle in clinical development—and a cause for therapeutic inefficacy. Without intraoperative monitoring, it has not been possible to determine how differences in surgical approaches, cannula designs, vector volumes, and dosing affect coverage within the brain (4).
One platform that allows for iMRI-guided catheter placement and real-time visualization of delivery of therapeutic agent is ClearPoint NeuroNavigation, developed by US-based medical device company, ClearPoint Neuro, which is currently enabling a range of clinical trials involving neuro-oncology, neurodegenerative disorders, rare genetic disorders, and movement disorders. It is an integrated hardware and software platform that communicates with the MRI scanner console to provide high-resolution imaging for trajectory planning, entry point selection, device placement, and infusion monitoring. The navigation system has been used in conjunction with the ClearPoint Neuro SmartFlow Cannula for precise dosage of drugs or gene therapies in approved clinical trials.
The introduction of iMRI-guided drug delivery uncovered many injection challenges associated with targeted infusion within the brain. Based upon the diffusion behaviors and characteristics observed during early iMRI monitoring in non-human primates, many protocols have now adopted a technique of either progressive advancement from proximal to distal for the duration of infusion, or a “pull-back” method for cell delivery where the first deposit is delivered to the most distal region. This technique of administering multiple infusions along the trajectory path was found to increase the surgeon’s ability to achieve infusions that cover the putamen or other target area as uniformly as possible, with the added benefit of providing the ability to move the cannula tip away from problematic perivascular spaces. In addition, innovations in cannula design have resulted in more reliable, efficient, and increased distribution of agents. Because image-guided systems provide real-time visualization of the infusion, they allow for intraoperative troubleshooting and real time modification to key infusion parameters, such as flow rate, positioning of the catheter, or terminating the infusion when the desired coverage has been attained (3).
This latter advancement is particularly noteworthy as it introduces the potential for specific patient dosing, a game-changing capability in treating CNS disorders or brain cancer. Historically, physicians have strictly followed a standardized trial protocol that specifies uniform infusion parameters across all patients, including the dose (volume) of therapeutic delivered. Evidence suggests that many early trials administered dosages that were insufficient to achieve desired clinical outcomes. iMRI-guided surgery offers the possibility of using much higher volumes, and adjusting the volume to focus on patient specific coverage, rather than a pre-determined amount. This concept of patient specific dosing is new and exciting in its potential to improve clinical outcomes—and it is currently being applied right now in an ongoing Parkinson’s Disease (PD) trial.
Finally, these technological advancements afford an improved level of safety. iMRI provides surgeons with a significantly higher degree of confidence that the cannula is following a safe trajectory and being placed in the desired target location. This is particularly important when targeting deep structures, where safe corridors through the brain can be only 2–3 millimeters wide.
Ongoing clinical trials of iMRI-guided drug delivery
At the University of California San Francisco, Paul Larson, MD, a professor and the vice-chair of Neurological Surgery at the university, is involved in three active human clinical programs utilizing iMRI-guided gene therapy delivery. The first is an amino acid decarboxylase program (AADC), which is an enzyme replacement gene therapy strategy for PD that uses adeno associated virus (AAV) to deliver a gene for AADC. The program, sponsored by Neurocrine Biosciences and Voyager Therapeutics, is now a Phase II multi-center study. The Phase I results indicate that, as coverage of the putamen improved, there was a significant increase in PET activity and a concurrent decrease in PD medications (5).
The second is a Phase Ib investigation sponsored by Brain Neurotherapy Bio to evaluate the safety and potential clinical effect of AAV2-GDNF delivered to the putamen in subjects with earlier stage PD. Growth factors are an exciting approach as they have the potential to be a disease-modifying therapy.
The third is the world’s first intracranial gene therapy study for Huntington’s Disease (HD). Sponsored by uniQure Biopharma, it is a Phase I/II study of AMT-130 delivered to the striatum of patients with early manifest HD and is designed to establish safety and proof-of-concept. The gene product is designed to interrupt production of the aberrant Huntington protein.
Beyond these studies, iMRI-guided delivery is being used in many other ongoing or planned Phase I and Phase II clinical trials for indications, including glioblastoma, PD, Huntington’s Disease, Amyotrophic Lateral Sclerosis (ALS), and pediatric indications, including AADC deficiency, Freidreich’s Ataxia, Angelman Syndrome, and Sanfillippo Syndrome. iMRI-guided delivery with the SmartFlow cannula is already cleared for Cytarabine injection into the ventricles to treat leukemia and non-Hodgkin's lymphoma, and numerous other programs are in development involving gene therapy, cell transplants, and other therapeutics.
Economic impact
Although it may be premature to assign hard savings to iMRI-guided drug delivery, there are significant potential benefits from such an approach. Given the tremendous R&D costs to develop a gene therapy as well as the cost of a single dose and the infusion procedure, any increase in the likelihood of a successful procedure would translate into considerable savings for the healthcare industry at large—as well as accelerating adoption of pharmaceutical gene therapies. The potential for iMRI guidance to ensure safe and accurate placement of the catheter, administer patient-specific dosages, minimize leakage, and optimize coverage addresses many of the challenges faced by early gene therapy trials. When considering the costs of past negative phase two trials, the business case for leveraging iMRI is apparent.
Advice for pharma
The advantages of real-time MRI-guided gene therapy delivery are clear. However, one challenge for pharma companies is the number of neurosurgery centers that have implemented iMRI at their facilities. The ClearPoint NeuroNavigation system is currently installed in more than 60 of the leading neurosurgical centers across the United States, and is expanding into Europe. While the number of institutions adopting iMRI is growing, there is a need for more pharmaceutical companies and neurosurgeons to embrace this improved delivery method for intracranial therapeutics. To do otherwise is to potentially jeopardize billions of dollars in R&D investments, put study participants in harm’s way with little chance to show benefit, and run the risk of obviating cures for life-threatening diseases.
References
1. J.P. Fuhr, “The Possibility of Encouraging Innovation and Competition by Erwin A. Blackstone.” www.semanticscholar.org (2015).
2. X. Dong, Theranostics 8 (6) 1481–1493 (2018).
3. S.J. Han, et al., Expert Rev Neurother. 16 (6) 635–639 (2016).
4. R.M. Richardson, et al., J Neurol Neurosurg Psychiatry91, 1210–1218 (2020).
5. C.W. Christine, et al., Ann Neurol. 85 (5) 704–714 (2019).
About the author
Paul S. Larson*, MD, paul.larson@ucsf.edu, is professor and vice-chair of Neurological Surgery at the University of California San Francisco, chief of Neurosurgery at the San Francisco Veterans Affairs Medical Center, and surgical director of the Parkinson’s Disease Research, Education and Clinical Center.
*To whom all correspondence should be addressed.