Investigational Use
in Approved Clinical Studies
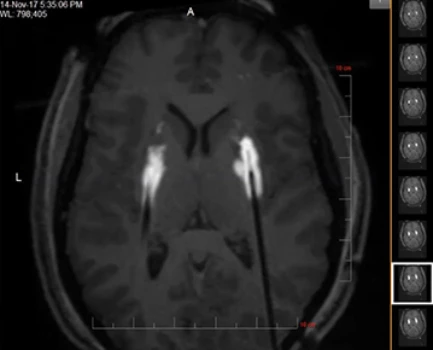
The ClearPoint Neuro Navigation Platform for Drug Delivery and Gene Therapy*
The FDA-cleared and CE Marked ClearPoint Neuro Navigation System provides real-time MRI guidance for cannula placement. When combined with the SmartFlow® Cannula*, this can allow for assessment of infusion coverage and delivery of approved therapeutics to regions of interest in the brain in clinical trials.
*For investigational use only in approved clinical trials. Please refer to the appropriate device labeling for further details.
See How The ClearPoint Neuro Navigation System Enables Drug Delivery
Benefits of ClearPoint-Enabled CNS Infusions*
*For investigational use only in approved clinical trials. Please refer to the appropriate device labeling for further details.
The ClearPoint Neuro Navigation System and the SmartFlow Cannula Have Enabled Clinical Trials Across the Following Indications
Parkinson’s | Huntingtons | Neuromuscular Disorders | Rare Genetic Disorders |
Epilepsy | Neuro-Oncology
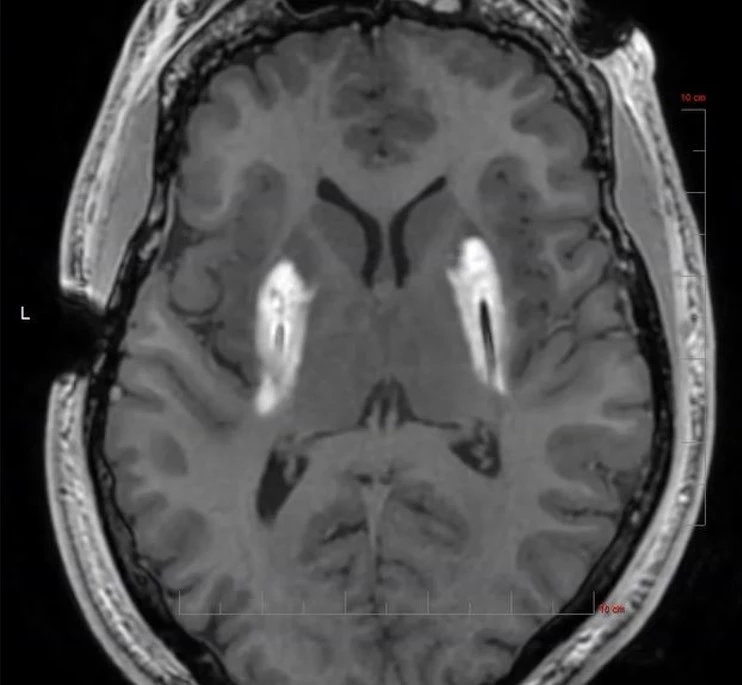
ClearPoint is Used to Deliver Biologics & Drugs in Clinical Trials With a Range of Partners, Including:
Featured ClearPoint Accessory
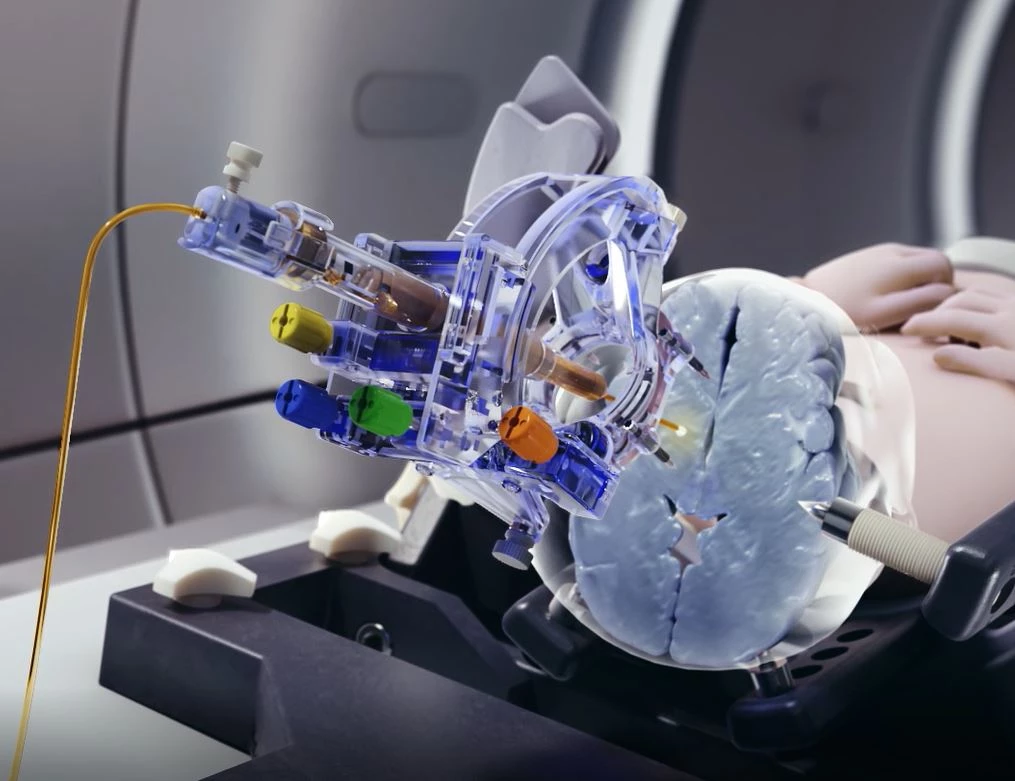
SmartFrame Array® Neuro Navigation System
Designed to streamline Laser Catheter Insertion, Drug Delivery, and Biopsy procedures – and enable more workflow options
The SmartFrame Array® offers stability and flexibility for neuro navigation, with a highly rigid frame and an “array” of six offset channels to simplify multi-trajectory procedures and entry point adjustments. The Array software provides an intuitive user interface, with options for performing entire procedures in the MRI, or starting in the operating room.
“The SmartFrame Array system offers a more rigid and compact build to enhance procedural efficiency while maintaining accuracy. The design supports multi-trajectory stereotaxis, allowing novel clinical applications.”
Clark C. Chen, MD, PhD
Professor, Lyle French Chair Neurosurgery & Department Head, Medical School
University of Minnesota Medical School
Educational Resources for Biologics & Drug Delivery
iMRI-Guided Gene Therapy and Drug Delivery for CNS Disorders
In this peer-reviewed publication, Dr. Paul Larson from UCSF discusses leveraging real-time MRI guidance for intracranial gene therapy administration and how it potentially improves efficacy and outcomes.
ClearPoint Bibliography
The ClearPoint Neuro Drug Delivery and Biologics Bibliography features ClearPoint being used in investigation and clinical research in many different peer-reviewed publications.
For any further questions on our educational resources, please reach out to our
Vice President of Translational and Pre-Clinical Research, Ernesto Salegio, PhD.