Biologics & Drug Delivery Partners
Simple Solutions for Navigating a Complex Landscape
ClearPoint Neuro strives to support our partners through all stages of therapy development, leveraging our FDA-cleared and CE marked MRI-guided stereotactic system for neurological interventions. We offer solutions that will guide you from the benchtop to pre-clinical studies, through clinical trials, and into post-commercialization, with translational continuity. Together, we will navigate the regulatory landscape with an industry-leading cadence of innovation and caliber of services–all fully customizable to meet your project needs.
If you have any questions regarding our biologics and drug delivery products or services, we’d love to hear from you:
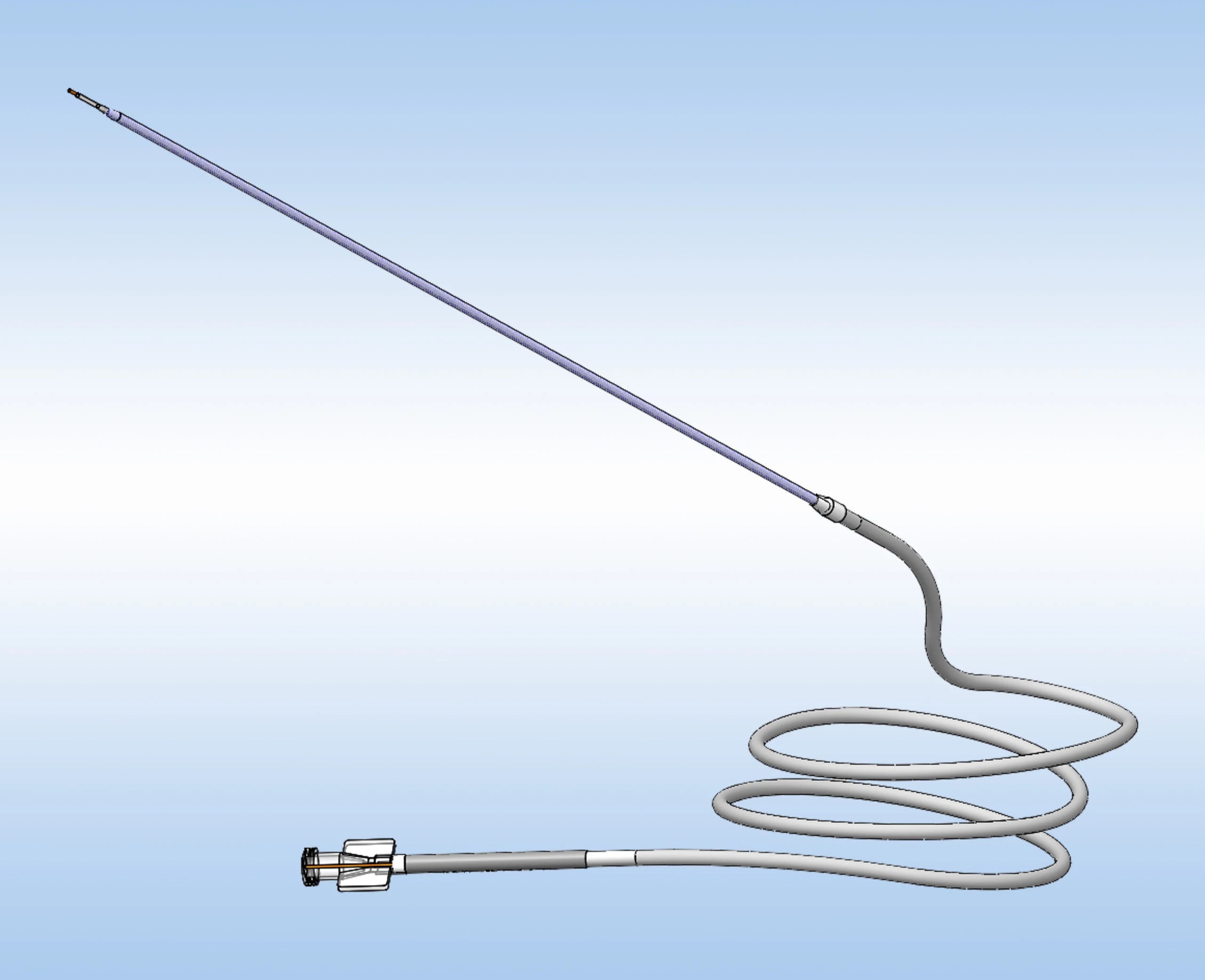
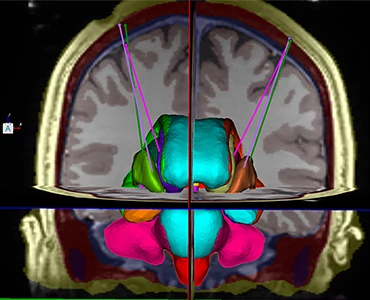
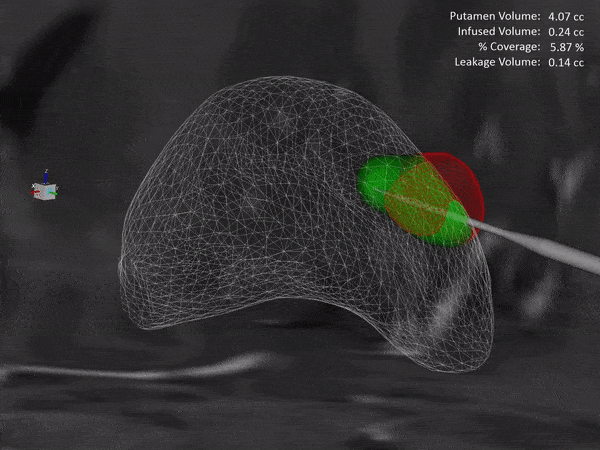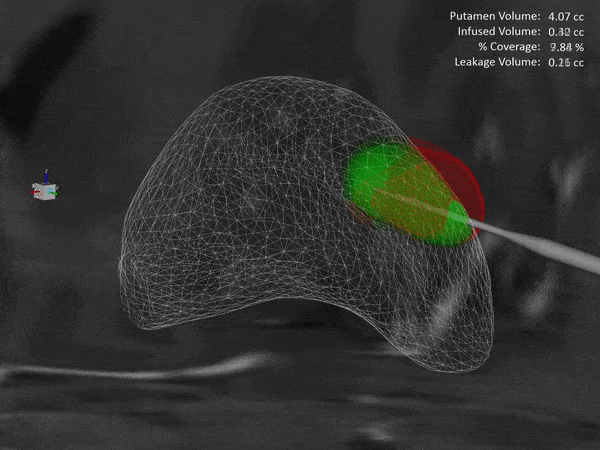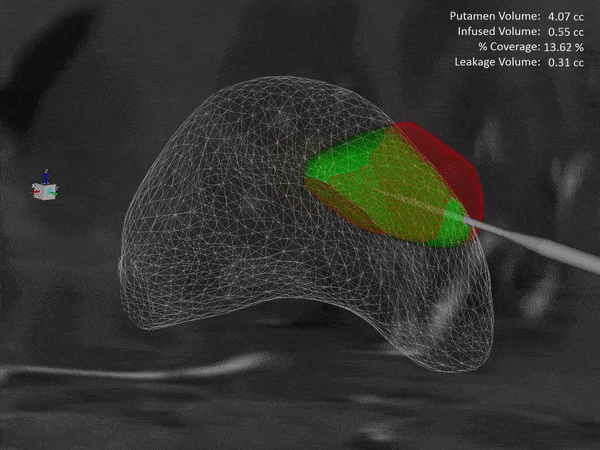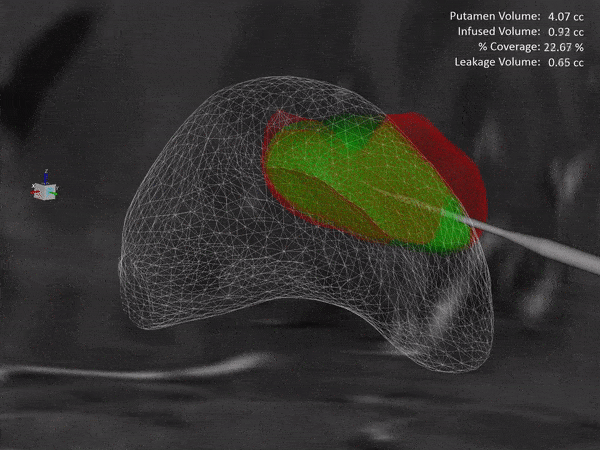
Learn how the ClearPoint® Navigation System and SmartFlow® Cannula can provide your pre-clinical studies and clinical trials with real-time confirmation of cannula placement and infusate coverage, while enabling intraprocedural adjustments to empower the delivery of drugs, stem cells, and gene therapy to regions of interest within the brain.
Stream Our Latest ClearPoint On-Demand Webinar:
Prone Positioning for iMRI: Optimizing Anesthesia Protocol
Featuring:
G. Rees Cosgrove, MD - Director, Epilepsy & Functional Neurosurgery / Director, Neurosurgical Residency Program
Dennis J. McNicholl, DO - Anesthesiologist
Dr. Rees Cosgrove and Dr. Dennis McNicholl, share their expertise on how to to access certain targets using prone positioning in the iMRI including how to optimize anesthesia protocols.
ClearPoint for MRI-Guided Delivery for Gene Therapy Clinical Trials:
ClearPoint Neuro Services & Solutions for Delivery of CNS Therapeutics in Pre-Clinical Studies & Clinical Trials
Learn more about how the ClearPoint team continues to expand our services and solutions for infusing gene therapies, cells, and other drugs in pre-clinical studies and clinical trials.
A child suffering from an extremely rare disease known as AADC deficiency successfully received gene therapy at the Policlinico Umberto I in Rome
The procedure for eladocagene exuparvovec therapy was outlined at a press conference organized by the Azienda Ospedaliero-Universitaria Policlinico Umberto I and Sapienza University of Rome in collaboration with Osservatorio Malattie Rare and with a non-conditional contribution from PTC Therapeutics. It was an eight-hour operation that involved the use of ClearPoint Neuro's SmartFlow® Cannula to enable the delivery of the therapy to the target points of the brain.
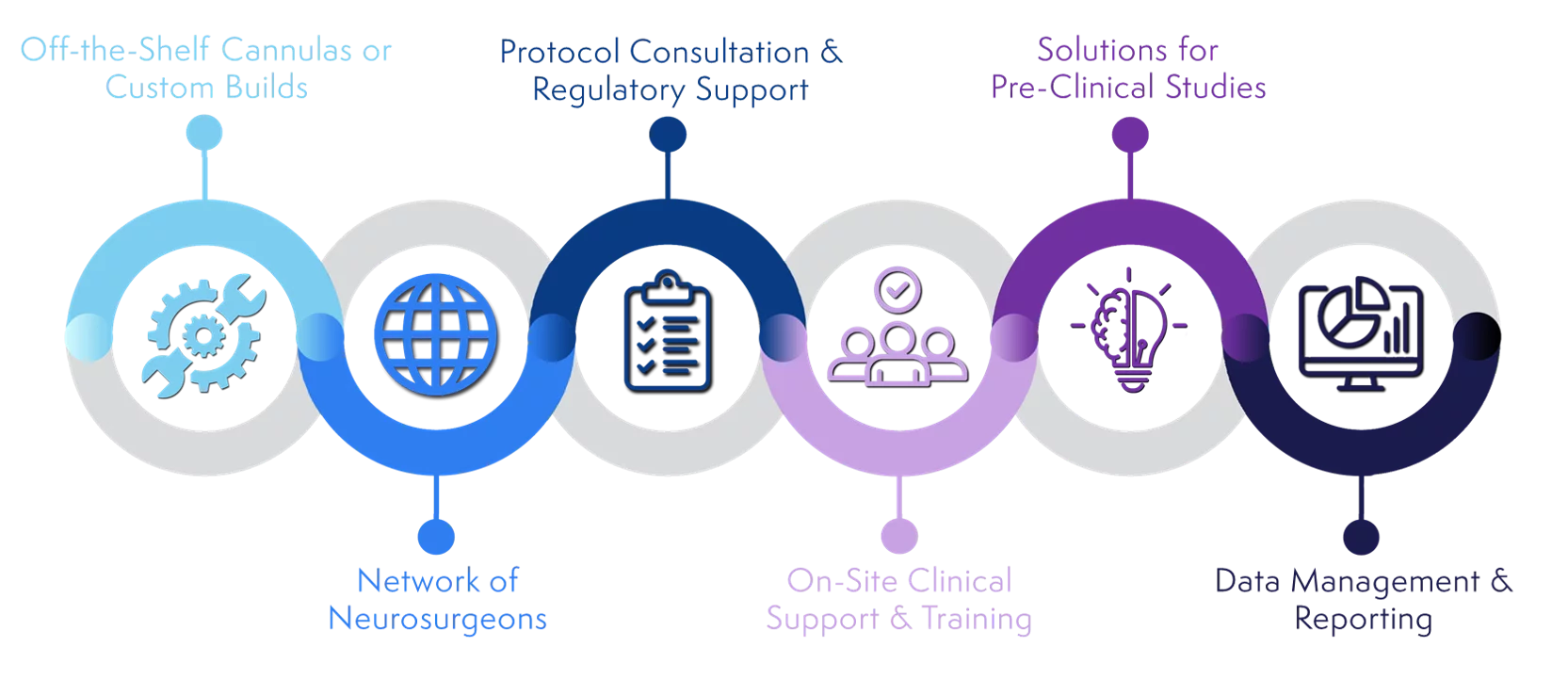
ClearPoint Neuro Biologics & Drug Delivery Solutions
ClearPoint Neuro Provides a Range of Solutions to
Meet the Needs of Your Clinical Trial
Reach out to our team to learn more about ClearPoint Neuro's comprehensive solutions that guide our partners from benchtop to pre-clinical studies, through clinical trials, and into post commercialization with an industry-leading cadence of innovation and caliber of services:
Featured ClearPoint Products
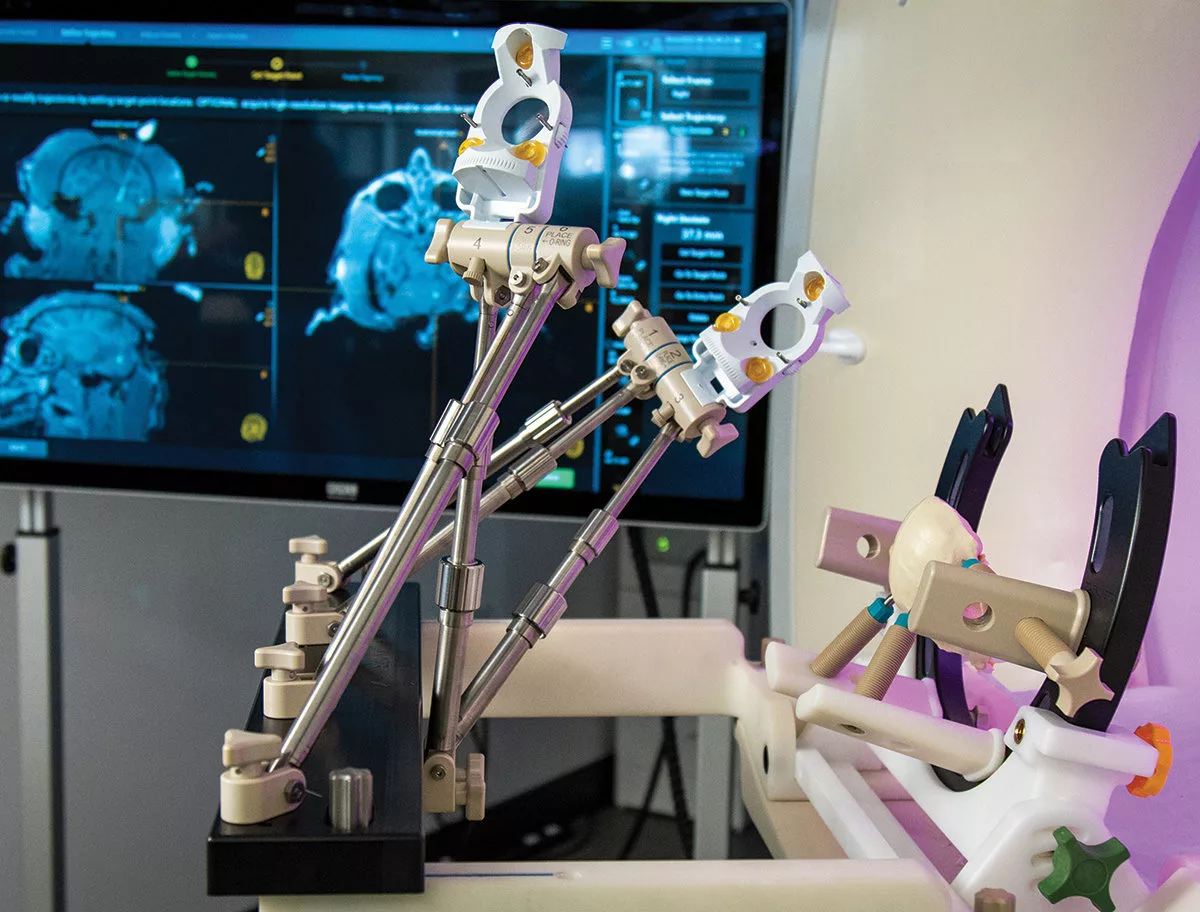
ClearPoint Pre-Clinical Orchestra™ Frame
Providing versatile fixation and positioning options for your pre-clinical surgical needs
The ClearPoint Pre-Clinical Orchestra™ provides study directors with versatile options for head pinning and navigation frame mounting in various pre-clinical models. The unique design of the head fixation component enables prone, supine, or lateral positioning, with flexibility for MRI imaging coil placement. By mimicking the clinical head fixation frame and utilizing the clinical navigation platform, the Orchestra allows for a seamless transition from pre-clinical research and studies into the clinic.
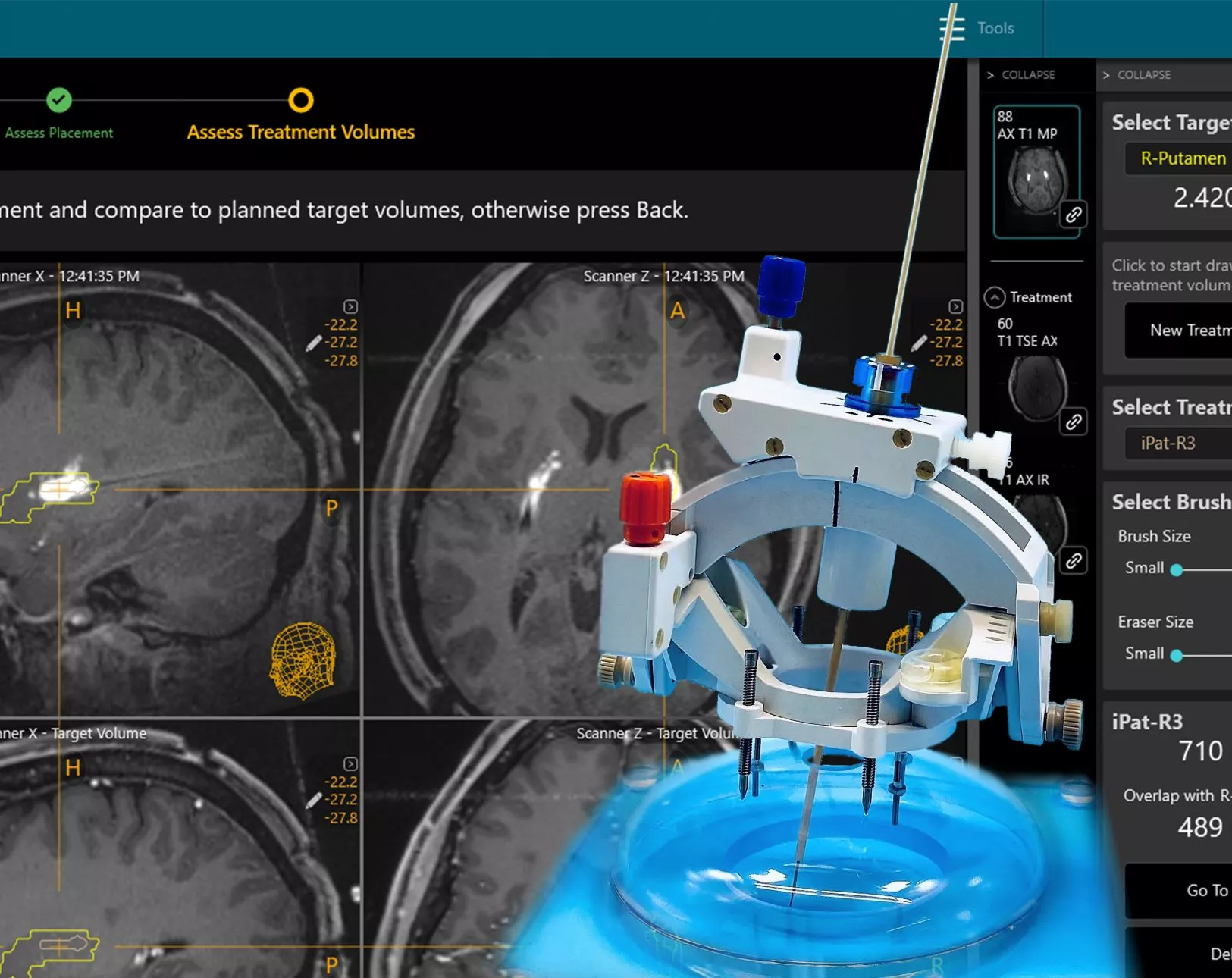
SmartFrame Array® Neuro Navigation System
Designed to streamline Drug Delivery procedures – and enable more workflow options
The SmartFrame Array® offers stability and flexibility for neuro navigation, with a highly rigid frame and an “array” of six offset channels to simplify multi-trajectory procedures and entry point adjustments. The Array software provides an intuitive user interface, with options for performing entire procedures in the MRI, or starting in the operating room.
Drug Delivery Development Projects
Radially Branching Intra-Cerebral Cellular Delivery Device
Multi-year licensing agreement of intellectual property from UC San Francisco (UCSF) through its Innovation Ventures group.
ClearPoint Neuro is working with UCSF to develop and commercialize an innovative radially branching cellular delivery device for use both in the Operating Room under fluoroscopy/CT guidance as well as MRI-guidance. This device is being developed to help clinicians guide the delivery of biologic therapies — from stem cell transplants to gene therapy vectors — to specific locations in the human brain by helping surgeons tailor biologic delivery to individual patient anatomy and specific diseases.
Gene Therapy Infusion Coverage Tool Integration into ClearPoint Maestro® Brain Model
Worldwide agreement with NE Scientific, LLC.
ClearPoint Neuro is working with NE Scientific, LLC to develop a GPU-accelerated software solution into the ClearPoint Maestro® Brain Model for modeling of drug infusions delivered with the ClearPoint SmartFlow® cannula. This software collaboration will enable simulation of patient-specific infusion intra-procedurally to predict the distribution of therapeutic agents in 4D before the actual procedure to allow optimization of infusion parameters including target points, flow rate, infusion coverage and leakage outside of target-specific boundaries.
Educational Resources for Biologics & Drug Delivery
ClearPoint On-Demand Webinar Discussing Gene Therapy Clinical Trials:
Data Driven Evolution of MRI-Guided Intra-Parenchymal Delivery for Gene Therapy Clinical Trials
Dr. Paul Larson, a leading gene therapy clinical trialist, discusses his experience with the data driven evolution of MRI-guided intra-parenchymal delivery for gene therapy clinical trials.
For any further questions on the pre-clinical studies and clinical trials, please reach out to our
Vice President of Translational and Pre-Clinical Research, Ernesto Salegio, PhD.