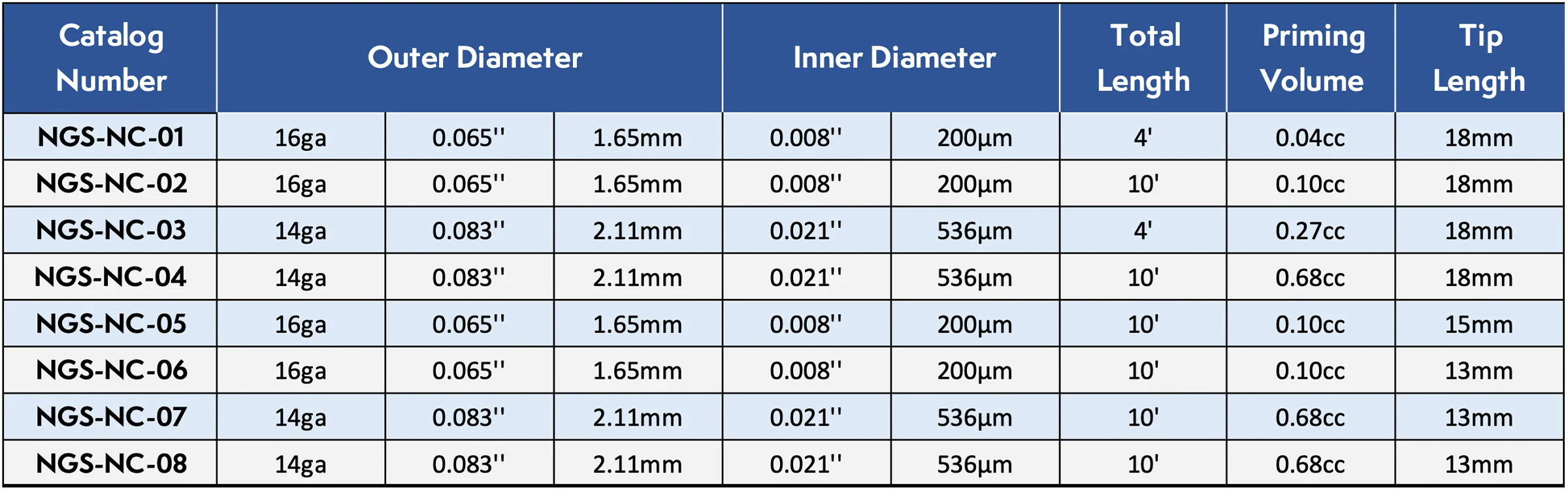
SmartFlow Neuro Cannula
The SmartFlow cannula uses Convection Enhanced Delivery (CED) to effectively deliver biologic therapies directly to regions of interest in the brain, bypassing the blood brain barrier.
- First device to be commercially approved in the US and Europe for delivery of gene therapy to the brain parenchyma.
- Enabling the delivery of 60+ investigational products, including gene and cell therapies.
The SmartFlow® Neuro Cannula has received 510(k) clearance from the FDA for use in the US for the aspiration of CSF, or injection of the chemotherapy drug Cytarabine into the ventricle. It has also been CE marked for use in Europe for the delivery of approved fluids into the brain or aspiration of CSF. It’s being utilized in approved clinical and preclinical studies for various research and drug trials. This device is not intended for implant and is intended for single patient use only.
With over 8,000 cannulas sold to-date, SmartFlow is
the industry-leading cannula for intraparenchymal drug delivery trials
- The SmartFlow Neuro Cannula can be used to bypass the blood brain barrier by delivering therapeutics to regions of interest.
- Used in trials to infuse gene therapy, viruses, or oncolytic agents via Convection Enhanced Delivery (CED), and deposit stem cells.
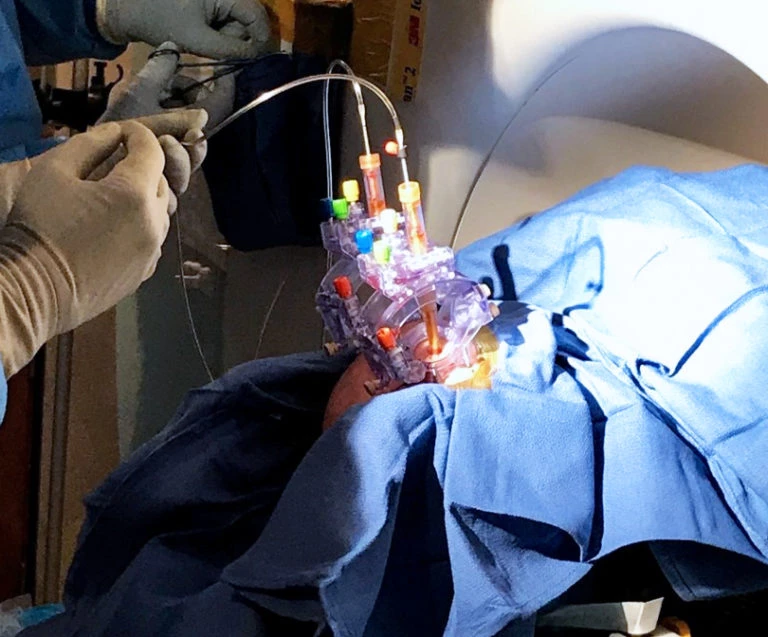
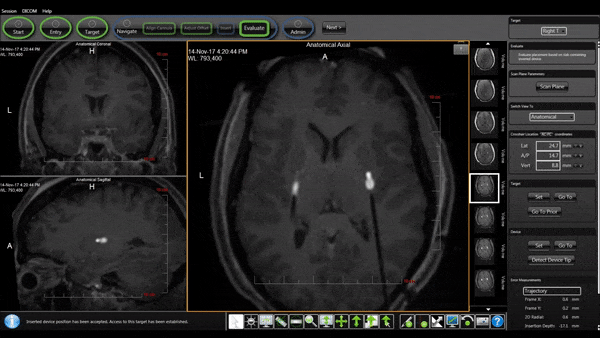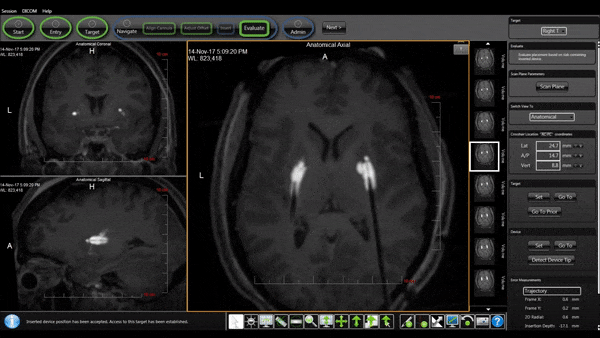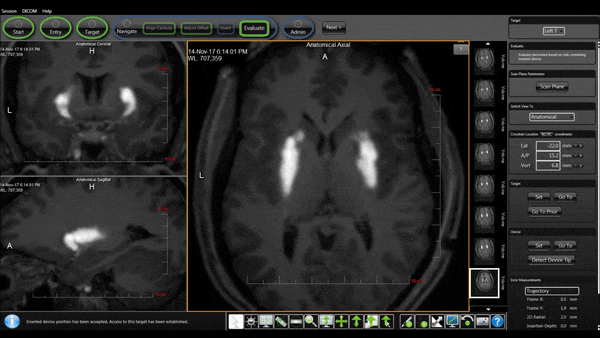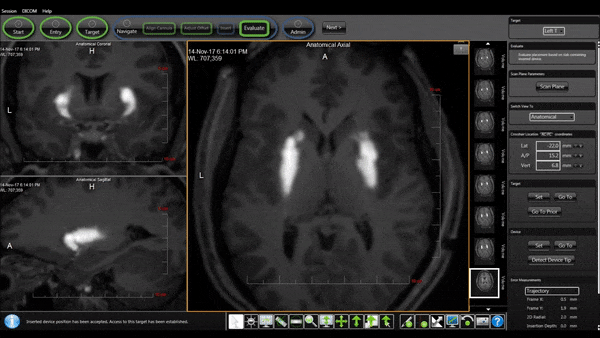
Bilateral Cannulas Allow for Simultaneous Infusion
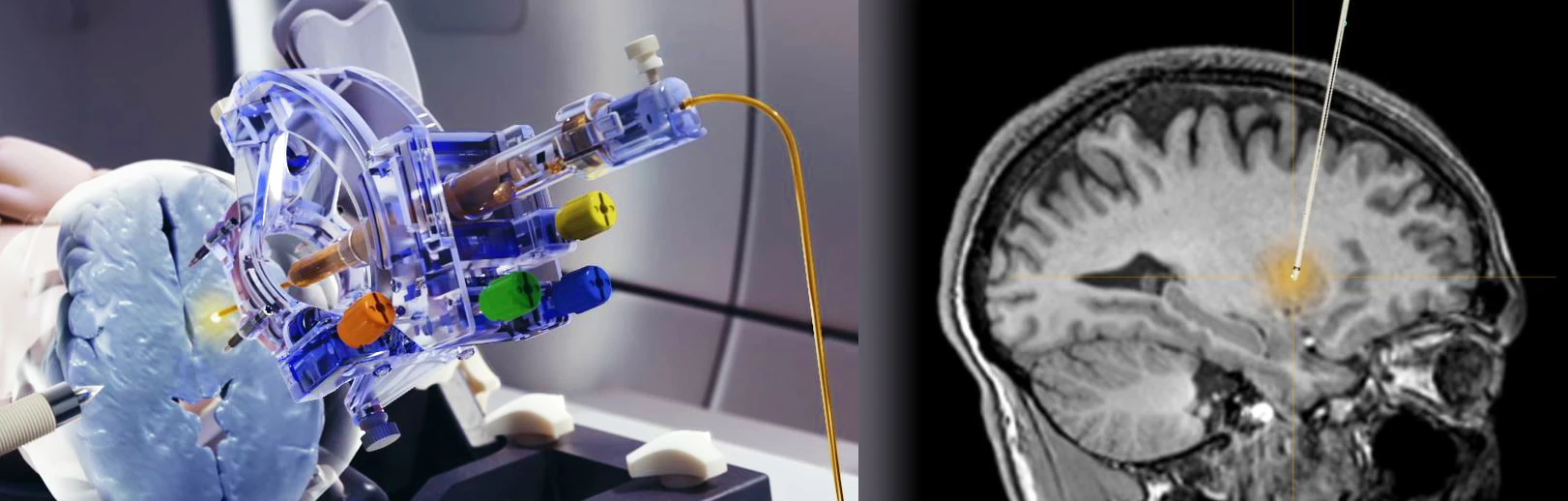
Why is MR Compatibility Important?
- MRI provides real-time confirmation of cannula placement and infusate coverage.
- Allows for accurate dosing to regions of interest in the brain.
- Enables intra-procedural adjustments to improve distribution.
- Allows for comparison of patient outcomes to infusion parameters.
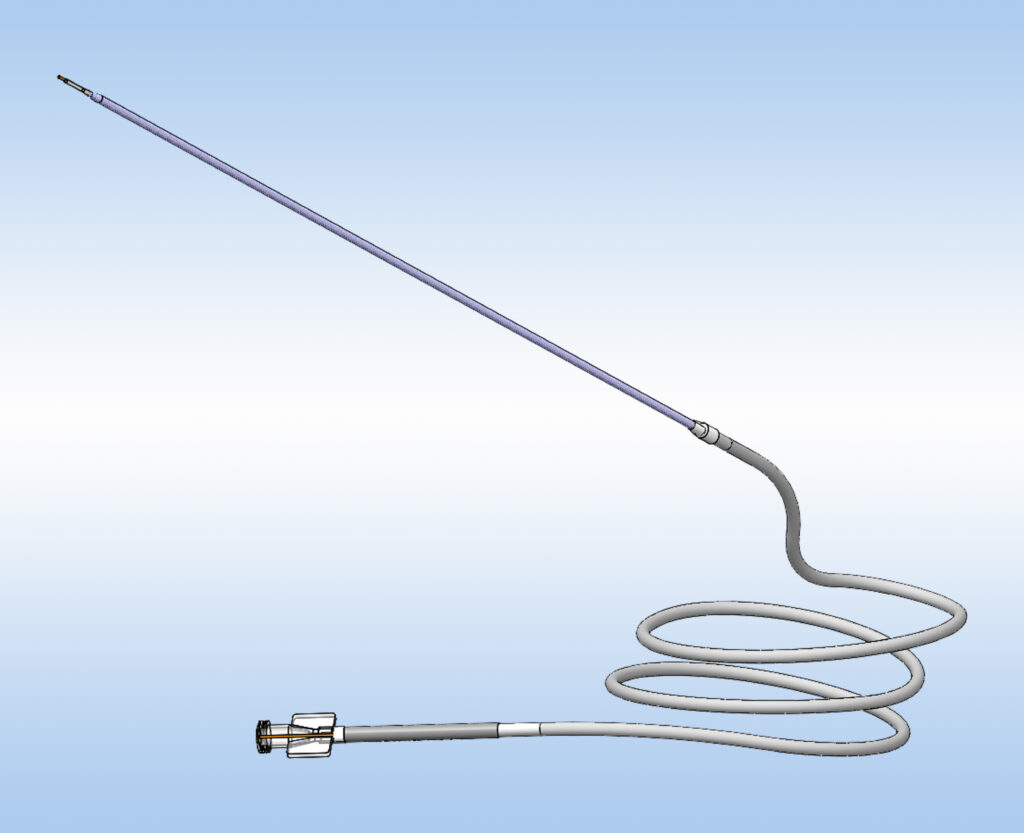
The stepped distal tip enables CED, and a single silica inner lumen allows for low volume priming, minimizes device interaction, and prevents leaks.

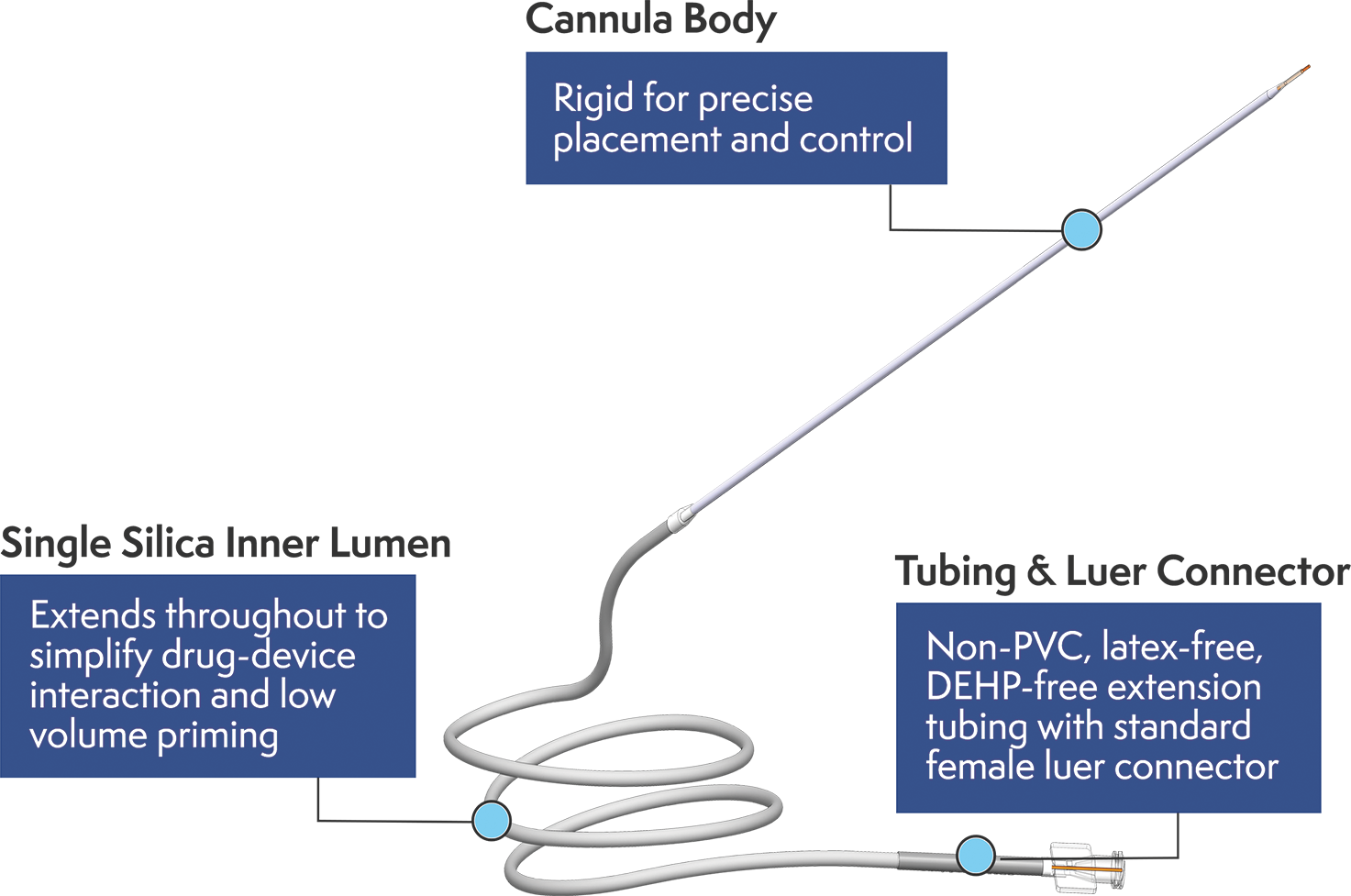

SmartFlow Neuro Cannula Benefits
- Integrated 4ft or 10ft tubing allow syringe/pump operation away from the patient and magnetic field.
- Multiple off-the-shelf configurations empower specific applications, and all parameters are customizable: