Intraparenchymal Delivery
Read more to learn about intraparenchymal delivery of therapeutics directly within the brain tissue, which bypasses the blood brain barrier.
The ClearPoint Biologics Blog is an educational tool for healthcare providers, clinical researchers, and our partners to provide the reader with information on different key topics. If you have any questions regarding our biologics and drug delivery products or services, we’d love to hear from you:
Intraparenchymal:
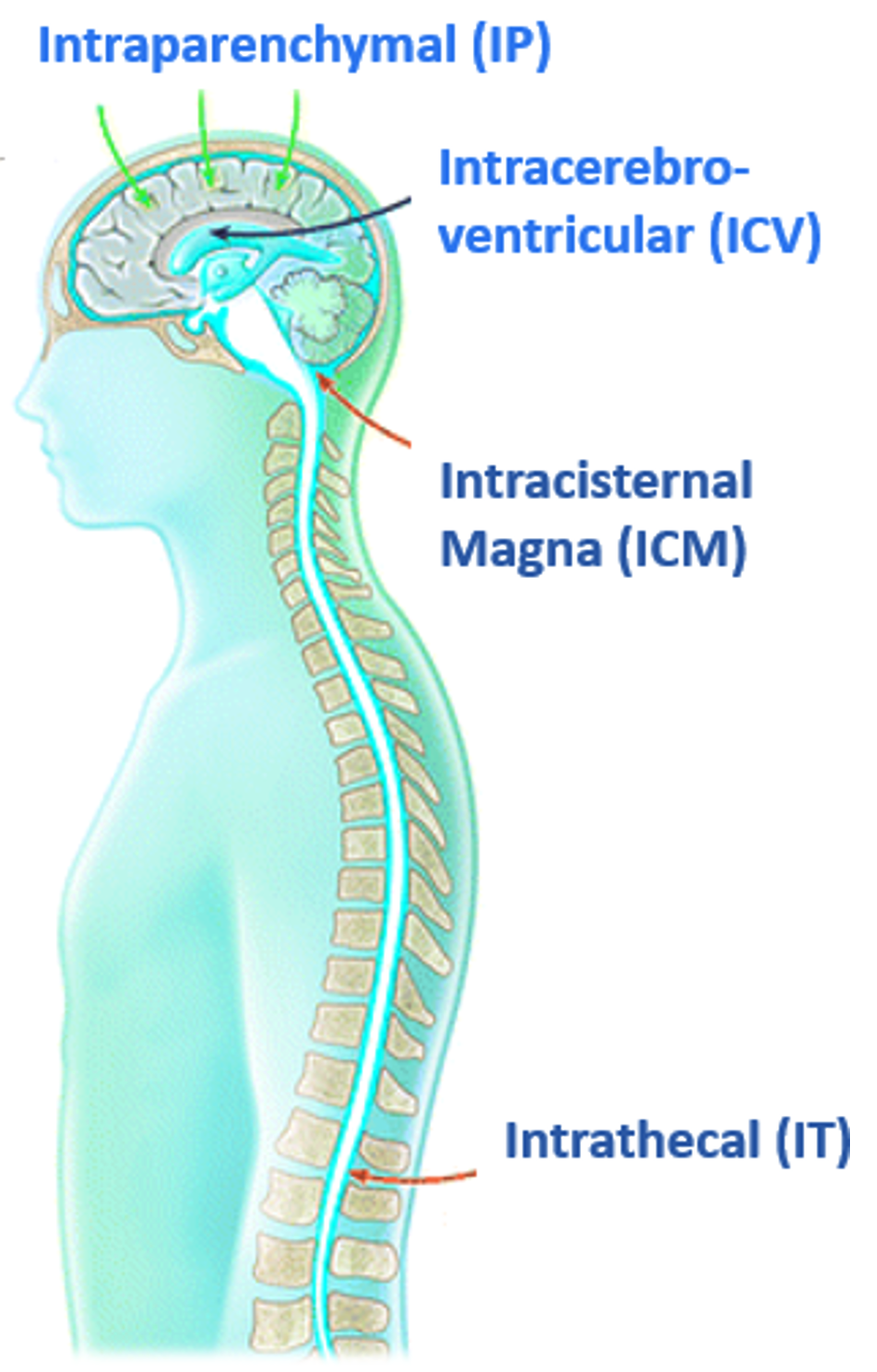
Common routes of administration (ROA) for therapeutics include intraoral (mouth), intramuscular (muscle), intravenous (vein), intrathecal (spinal canal), intracisternal (cisterna magna), and intracerebroventricular (ventricles). With regards to therapies designed for neurological disorders or neurodegenerative disease, these “systemic” ROA’s typically require higher doses to achieve any therapeutic benefit and have been shown to cause systemic toxicity which can impede dose escalation to therapeutic levels (Tosi & Souweidane, 2020). “Intraparenchymal” refers to delivery of therapeutics directly within the brain tissue which bypasses the blood brain barrier (BBB) altogether and has been shown to allow for larger volumes of infusate to be delivered while limiting neuro and systemic toxicity (Vogelbaum & Aghi, 2015; Han et al., 2016).
Image adapted from Hocquemiller M, et al. Adeno-Associated Virus-Based Gene Therapy for CNS Disease. Hum Gene Ther. 2016: 478-496 and modified to demonstrate some of the routes of administration for therapeutics. doi:10.1089/hum.2016.087
ClearPoint’s MRI-guided neurosurgical platform specializes in the placement of devices (DBS electrodes, biopsy needles, laser ablation catheters and cannulas) in the brain with submillimetric accuracy (Richardson et al., 2020). By having the ability to continuously update the soft tissue information provided by MRI; brain shift, hemorrhage, or any issues with the delivery of a therapeutic can be identified allowing for necessary adjustments to be made in real-time (Chittiboina et al., 2015; Jahangiri et al., 2017; Richardson et al., 2020; Salegio et al., 2022; Chadwick et al., 2019; Rowland et al., 2016; Richardson et al., 2011; Silvistrini et al., 2015; Rossmeisl et al., 2021; Buttery & Barker, 2020).
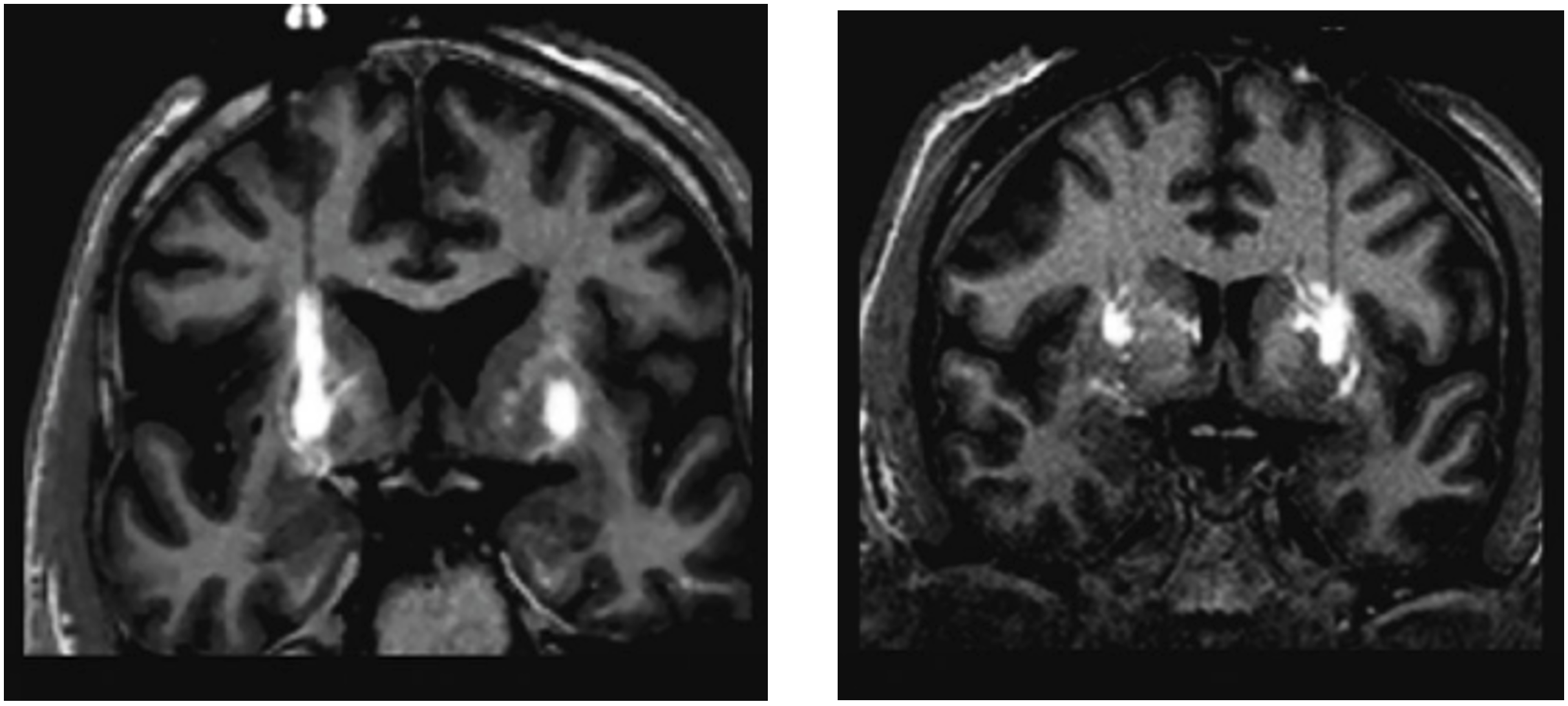
Image adapted from Richardson, R. M., Bankiewicz, K. S., Christine, C. W., Van Laar, A. D., Gross, R. E., Lonser, R., Factor, S. A., Kostyk, S. K., Kells, A. P., Ravina, B., & Larson, P. S. (2020). Data driven evolution of neurosurgical gene therapy delivery in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry, 91(11), 1210–1218. https://doi.org/10.1136/jnnp-2020-322904 to demonstrate “reflux” or “back-flow” (LEFT) as well as “perivascular spread” (RIGHT).
References:
- Tosi, U., & Souweidane, M. (2020). Convection Enhanced Delivery for Diffuse Intrinsic Pontine Glioma: Review of a Single Institution Experience. Pharmaceutics, 12(7), 660. https://doi.org/10.3390/pharmaceutics12070660
- Han SJ, Bankiewicz K, Butowski NA, Larson PS, Aghi MK. Interventional MRI-guided catheter placement and real time drug delivery to the central nervous system. Expert Review of Neurotherapeutics. 2016;16(6):635–639. doi:10.1080/14737175.2016.1175939
- Vogelbaum, M. A., & Aghi, M. K. (2015). Convection-enhanced delivery for the treatment of glioblastoma. Neuro-oncology, 17 Suppl 2(Suppl 2), ii3–ii8. https://doi.org/10.1093/neuonc/nou354
- Richardson MR, Bankiewicz KS, Chadwick CW, Van Laar AD, et al. Data-driven evolution of neurosurgical gene therapy delivery in Parkinson’s disease. Journal of Neurology, Neurosurgery, & Psychiatry. 2020. doi:10.1136/jnnp-2020-322904
- Chittiboina, P., Heiss, J. D., & Lonser, R. R. (2015). Accuracy of direct magnetic resonance imaging-guided placement of drug infusion cannulae. Journal of neurosurgery, 122(5), 1173–1179. https://doi.org/10.3171/2014.11.JNS131888
- Jahangiri, A., Chin, A. T., Flanigan, P. M., Chen, R., Bankiewicz, K., & Aghi, M. K. (2017). Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. Journal of neurosurgery, 126(1), 191–200. https://doi.org/10.3171/2016.1.JNS151591
- Salegio EA, Cukrov M, Lortz R, Green A, et al. Feasibility of Targeted Delivery of AAV5-GFP into the Cerebellum of Nonhuman Primates Following a Single Convection-Enhanced Delivery Infusion. Human Gene Therapy. 2022;3: Epub ahead of print. doi:10.1089/hum.2021.163
- Chadwick CW, Bankiewicz KS, Van Laar AD, Richardson MR, et al. Magnetic resonance imaging–guided phase 1 trial of putaminal AADC gene therapy for Parkinson's disease. Annals of Neurology. 2019;85:704–714. doi:10.1002/ana.25450
- Rowland NC, Kalia SK, Kalia LV, Larson PS, et al. Merging DBS with viral vector or stem cell implantation: "Hybrid" stereotactic surgery as an evolution in the surgical treatment of Parkinson's disease. Molecular Therapy Methods & Clinical Development. 2016;3:15051. doi:10.1038/mtm.2015.51
- Richardson MR, Kells AP, Martin AJ, Larson PS, et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotactic and Functional Neurosurgery. 2011;89(3):141–151. doi:10.1159/000323544
- Silvestrini MT, Yin D, Martin AJ, Coppes VG, et al. Interventional magnetic resonance imaging-guided cell transplantation into the brain with radially branched deployment. Molecular Therapy Methods & Clinical Development. 2015;23(1):119–129. doi:10.1038/mt.2014.155
- Rossmeisl JH, Herpai D, Quigley M, Cecere TE, et al. Phase I trial of convection-enhanced delivery of IL13RA2 and EPHA2 receptor targeted cytotoxins in dogs with spontaneous intracranial gliomas. Neuro-Oncology. 2021;23(3):422-434. doi:10.1093/neuonc/noaa196
- Buttery PC, Barker RA. Gene and cell-based therapies for Parkinson’s disease: Where are we? Neurotherapeutics. 2020;17:1539-1562. doi:10.1007/s13311-020-00940-4
ClearPoint Neuro Biologics & Drug Delivery Solutions
Simple Solutions for Navigating a Complex Landscape
ClearPoint Neuro strives to support our partners through all stages of therapy development, leveraging our FDA-cleared and CE marked MRI-guided stereotactic system for neurological interventions. We offer solutions that will guide you from the benchtop to pre-clinical studies, through clinical trials, and into post-commercialization, with translational continuity. Together, we will navigate the regulatory landscape with an industry-leading cadence of innovation and caliber of services–all fully customizable to meet your project needs.
If you have any questions regarding our biologics and drug delivery products or services, we’d love to hear from you: