From Joe Burnett, President & CEO:
As we move toward the post-pandemic era, we are proud to share many wonderful recent ClearPoint Neuro achievements with you, our customers and partners.
Clinically, we have seen a restoration to near pre-pandemic levels of elective procedures at hospitals across the United States. In Europe, despite remaining travel restrictions, our team has also been busy visiting a number of centers, including in Warsaw, Poland to prepare for upcoming Site Initiation Visits for biologics and drug delivery trials.
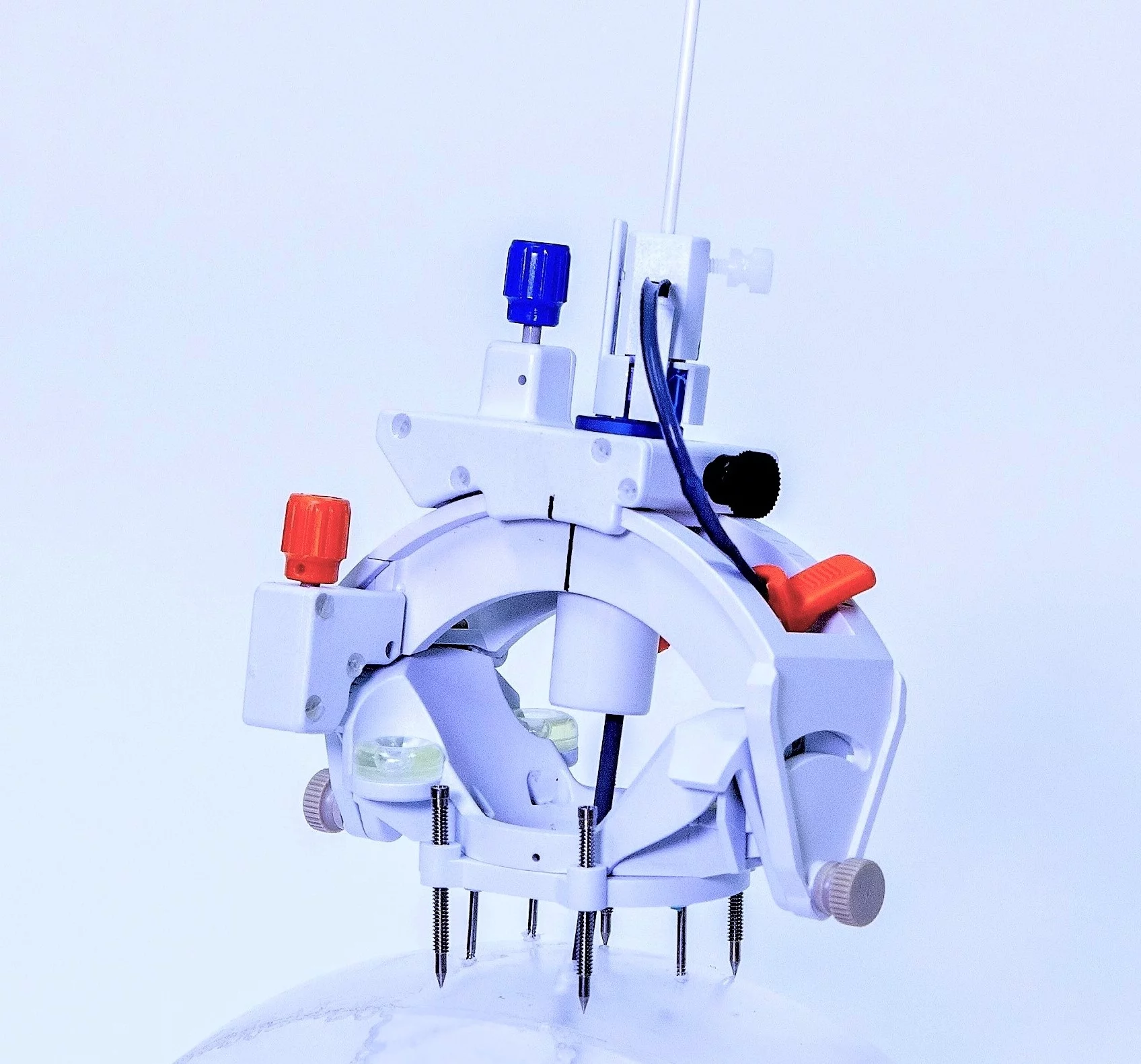
We have also experienced a number of ClearPoint 'firsts' this past quarter. Last month Dr. Clark Chen from the University of Minnesota Medical Center performed the first-in-human cases with our next generation SmartFrame Array hardware and software system, currently in limited market release. Array is an exciting innovation because it signifies our expansion beyond the MRI suite into the operating room, where the majority of neurosurgical procedures still take place. Additionally, earlier this month our team supported the first all-ClearPoint delivery into the brain of stem cell-derived dopaminergic neurons for a Phase I clinical trial in patients with advanced Parkinson's disease sponsored by BlueRock Therapeutics. And finally, in May, we announced an important partnership with D&K Engineering to develop our first robotic platform, designed for both the MRI suite and OR.
We also have exciting events planned this summer, including the grand opening of our new Training and Innovation Center in Solana Beach, California where we plan to host Fellows training programs, Medical Advisory Boards, pharmaceutical partners and investor meetings. In case you missed the sneak peek of our new location, check it out here.
Thank you for your ongoing support of our mission and team. We can't wait to see you all in person again soon.
First-in-Human SmartFrame Array™ Neuro Navigation System and Software Cases - Not Commercially Available in All Countries
Last month we announced the first-in-human procedures performed with the SmartFrame Array Neuro Navigation System & Software. Initial cases helped Dr. Clark Chen at the University of Minnesota Medical Center complete three procedures using ClearPoint in a single day, showing the potential of Array to shorten procedure times. Click Here to Learn More
ClearPoint Partners with D&K Engineering to Develop Robotic System for use in the MRI Suite & OR
ClearPoint Neuro's partnership with San Diego-based D&K Engineering empowers the development of a robotic system for use in both the MRI suite and operating room. The partnership enables an efficient pathway to innovation by automating specific parts of the planning and navigation during neurosurgery, allowing neurosurgeons to focus on the most crucial and exacting parts of the procedure. Click Here to Read More
Visit the ClearPoint Neuro Virtual Booth at the 2nd Annual Gene Therapy for Neurological Disorders EU Digital Event
ClearPoint will be virtually exhibiting at the 2nd Annual Gene Therapy for Neurological Disorders Europe Conference, running from July 19th – 21st. Megan S. Keiser, PhD, Project Manager of Translational Neuroscience & Director of Laboratory Research at the Children's Hospital of Philadelphia, will be presenting on Pre-Clinical Intraparenchymal Applications for Gene Therapies in the Non-Human Brain at 8:20AM EST on Wednesday, July 21st. Click Here to Learn More
BlueRock Therapeutics Utilizes ClearPoint Technology to Administer First Dose of DA01 Stem Cell Therapy for Phase I Study in Patients with Parkinson's Disease
Congratulations to partner, BlueRock Therapeutics, on the first patient dosed with DA01 in Phase 1 Study of patients with advanced Parkinson’s Disease. This marks the first ever stem cell clinical trial patient to be treated using the entire ClearPoint platform including two SmartFrames & six SmartFlow cannulae. The procedure was performed by Dr. Viviane Tabar, Chair of the Department of Neurosurgery & Theresa Feng Chair in Neurosurgical Oncology at Memorial Sloan Kettering Cancer Center. Click Here to Learn More
ClearPoint Neuro Congratulates Voyager Therapeutics on FDA Clearance of IND Application for Gene Therapy Candidate VY-HTT01 for the Treatment of Huntington's Disease
The ClearPoint team congratulates our partner, Voyager Therapeutics, on receiving FDA clearance of the IND application for their gene therapy candidate VY-HTT01 for the treatment of Huntington’s disease. We are thrilled to continue supporting Voyager’s clinical program development with ClearPoint products and clinical services for this important planned Phase 1/2 trial. Click Here to Read More
Visit ClearPoint Neuro's New Website
We are excited to announce the launch of our newly re-designed and modernized website. Visit www.clearpointneuro.com to learn more about ClearPoint's dedication to innovation in the neuro space. Click Here to Visit Our New Site