From Joe Burnett, President & CEO:
The closing of one year and opening of another provides an important interlude to take stock, and celebrate our team's 2021 accomplishments.
On the clinical front, last month, ClearPoint's global clinical specialist team supported our 5,000th procedure, only 11 months after completing our 4,000th. We are both humbled and honored to serve as an extension of our customers' teams, be they Neurosurgeons, MRI technologists, OR nurses, scrub techs, hospital administrators, or pharmaceutical partners.
With regards to our innovation pipeline, we have put funds from our February 2021 capital raise to good use ensuring all development activities are on track. A key component to our innovation strategy is to involve a diverse group of Neurosurgeons in our product development process. In Q4, we have capitalized on the return of in-person medical conferences to obtain important hands-on feedback and insights on our pipeline.
In Q1 of this year we set a goal to exit the COVID-19 pandemic in a better position than we started. Although a definitive exit seems elusive, I am proud to see our team continue to relentlessly execute on our strategy, partnership development, and portfolio expansion.
Wishing you and yours a very happy and safe holiday season. We look forward to a tremendous 2022!
Introducing the ClearPoint Champions Patient Education Series
At ClearPoint Neuro, we always put patients first. We're excited to introduce the first installment of our new ClearPoint Champions series. Susan, a patient from Hackensack Medical Center, shares her MRI-guided DBS surgical journey after being diagnosed with Parkinson's in 2015. We hope these stories serve as a powerful resource for patients and families who want to learn more about surgical options empowered by ClearPoint technology. Click Here to Watch
ClearPoint Neuro Congratulates Partner Blackrock Neurotech on Receiving Breakthrough Device Designation from the FDA for the MoveAgain Brain-Computer Interface System
The MoveAgain BCI System is expected to provide immobile patients the ability to control a cursor, keyboard, mobile device, wheelchair or prosthetic device simply by thinking. ClearPoint is proud to leverage our 20+ years of experience innovating novel neuro-navigation platforms and software with the aim of developing a new surgical solution that will allow neurosurgeons to implant Blackrock’s BCIs in the operating room. Click Here to Read More
Linda M. Liau, MD, PhD, MBA Joins ClearPoint Neuro Board of Directors
We are delighted to announce that internationally-renowned neurosurgeon and accomplished researcher, Dr. Linda M. Liau, has joined our Board of Directors. We look forward to the contributions she will make to our company and strategy, especially given her direct experience in neuro-based biologics and drug delivery. Click Here to Read More
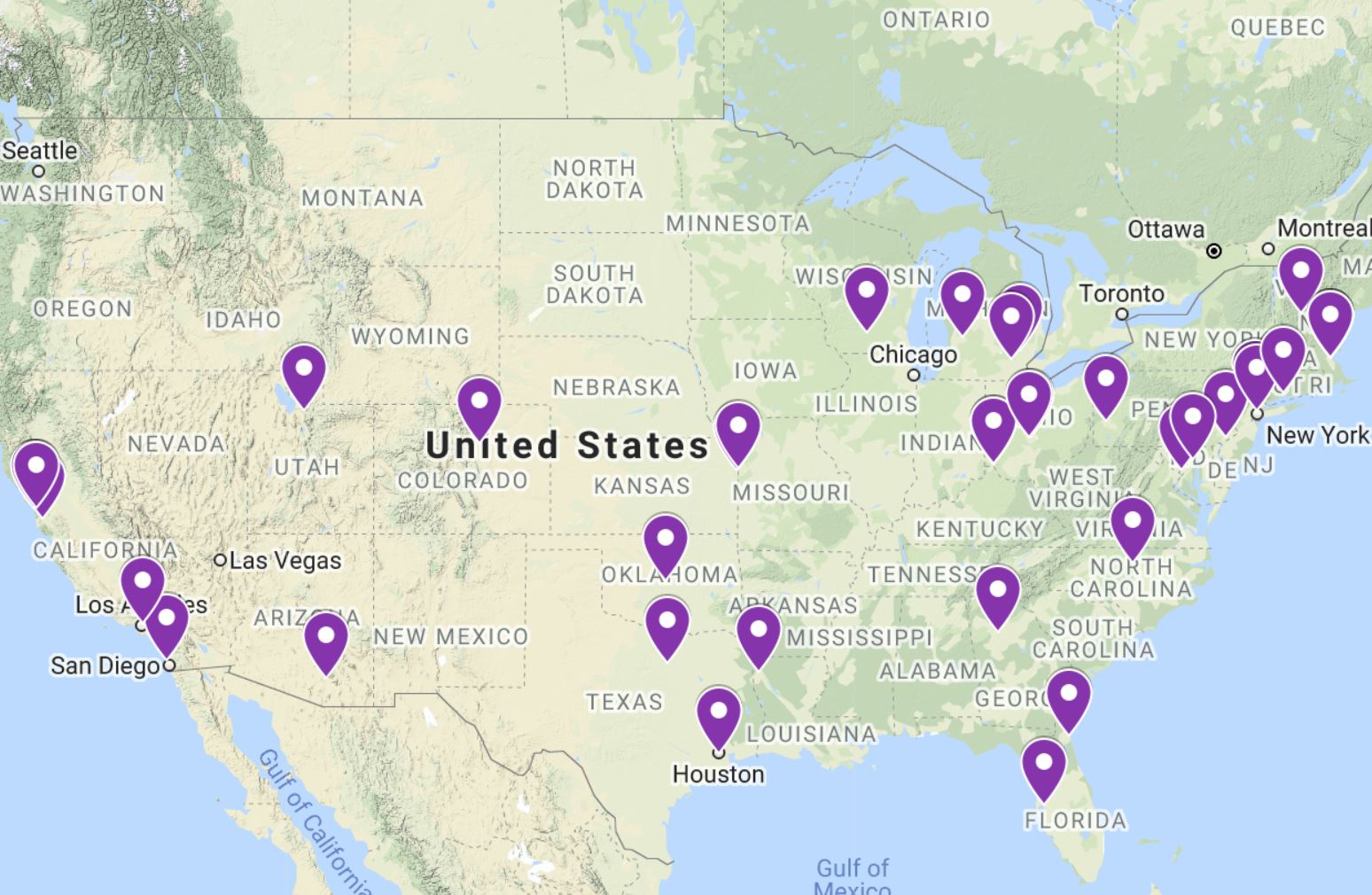
Introducing ClearPoint Neuro's MRI-Guided Deep Brain Stimulation Center Locator for Patients
In our continuing effort to provide patients and their families with new educational resources, we're pleased to introduce a locator of ClearPoint MRI-Guided DBS Centers for patients. This locator lists all sites in the United States that provide options for MRI-Guided Deep Brain Stimulation (DBS) under general anesthesia with ClearPoint. Click Here to Check it Out
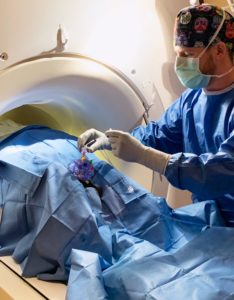
ClearPoint Neuro Supports 5,000th Procedure
Last month, we supported our 5,000th ClearPoint Neuro Navigation procedure. This milestone is a testament to the work of our entire team of Clinical Specialists, as well as our operations and manufacturing teams. We are proud that our technology has empowered neurosurgeons to deliver a variety of therapies to treat the most complex neurological diseases and disorders. We look forward to supporting another 5,000+ procedures globally in the coming years. Click Here to Learn More About ClearPoint Neuro Navigation System
ClearPoint Neuro Congratulates Our Partner Neurona Therapeutics on IND Clearance for Neural Cell Therapy NRTX-1001 in Chronic Focal Epilepsy Patients
Congratulations to our partner, Neurona Therapeutics, on their IND clearance for neural cell therapy NRTX-1001 in chronic focal epilepsy patients. We are very excited to be working with Neurona on potentially the first human cell therapy candidate to enter clinical trials for epilepsy. The potential of an expanded treatment option will provide additional hope for millions of patients worldwide. Click Here to Learn More