From Joe Burnett, President & CEO:
Welcome to our Q2 newsletter. As you’ll see below, we have lots of terrific news to share.
I'd like to start with news from our Biologics and Drug Delivery business. As we cautiously move into a post-pandemic reality, many of our pharmaceutical partners have begun actively enrolling patients into clinical trials in several geographies and achieved some exciting milestones:
- In May, our partner Lysogene shared a clinical update for their AAVance phase 2/3 clinical trial for gene therapy LYS-SAF302 for the treatment of MPS IIIA. The promising efficacy data showed a persistent increase or stabilization in 3 main developmental domains (cognition, motor and language) in all patients enrolled under the age of 30 months.
- Also last month, PTC Therapeutics received a positive opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommending its gene therapy treatment Upstaza™ (eladocagene exuparvovec) for the treatment of aromatic L-amino acid decarboxylase (AADC) deficiency. ClearPoint’s proprietary CE Marked SmartFlow® Neuro Ventricular Cannula is included in the submission for minimally invasive infusion of the Upstaza gene therapy. This significant milestone marks the first ever positive regulatory recommendation anywhere in the world for direct injection of a gene therapy to the brain.
- Last week uniQure announced encouraging safety and biomarker data from 10 patients enrolled in their Phase I/II clinical trial of AMT-130 for the treatment of Huntington's Disease.
- And finally, yesterday, Neurona Therapeutics announced the dosing of their initial patient in their Phase 1/2 clinical trial to treat drug-resistant mesial temporal lobe epilepsy.
Our team has been executing relentlessly to support both our neurosurgery and pharmaceutical customers across the globe, We recently announced receipt of Medical Device Single Audit Program certification (MDSAP). MDSAP allows a single regulatory audit of our quality management system to satisfy the regulatory requirements of multiple countries: Australia, Brazil, Canada, Japan and the United States. MDSAP, in addition to the 10 additional offers of employment we extended this quarter, will continue to power our global expansion, supporting our innovation pipeline, and commercial approvals beyond the US and EU.
Thank you for your continued support of our vision and our team. If you have any questions for me, please do not hesitate to reach out.
Calling Neurosurgical Fellows/Residents (PGY-3+): Reserve Your Spot in our Upcoming 2022 Clear Education Fellowship Courses
We invite Neurosurgical Fellows or Residents (PGY-3 and above) to join us at our Solana Beach, CA Global Training & Innovation Center to gain hands-on practical knowledge of iMRI-guided surgical techniques from luminary Functional Neurosurgeons. Space is limited so please sign-up today. Complimentary economy travel to be arranged by ClearPoint Neuro.
Aug. 19-21, 2022 (Friday - Sunday)
Faculty: Jessica A. Wilden, MD
Sept. 16-18, 2022 (Friday - Sunday)
Faculty: R. Mark Richardson, MD, PhD
Dec. 9-11, 2022 (Friday - Sunday)
Faculty: Paul S. Larson, MD, FAANS
Click Here to Submit Your Application
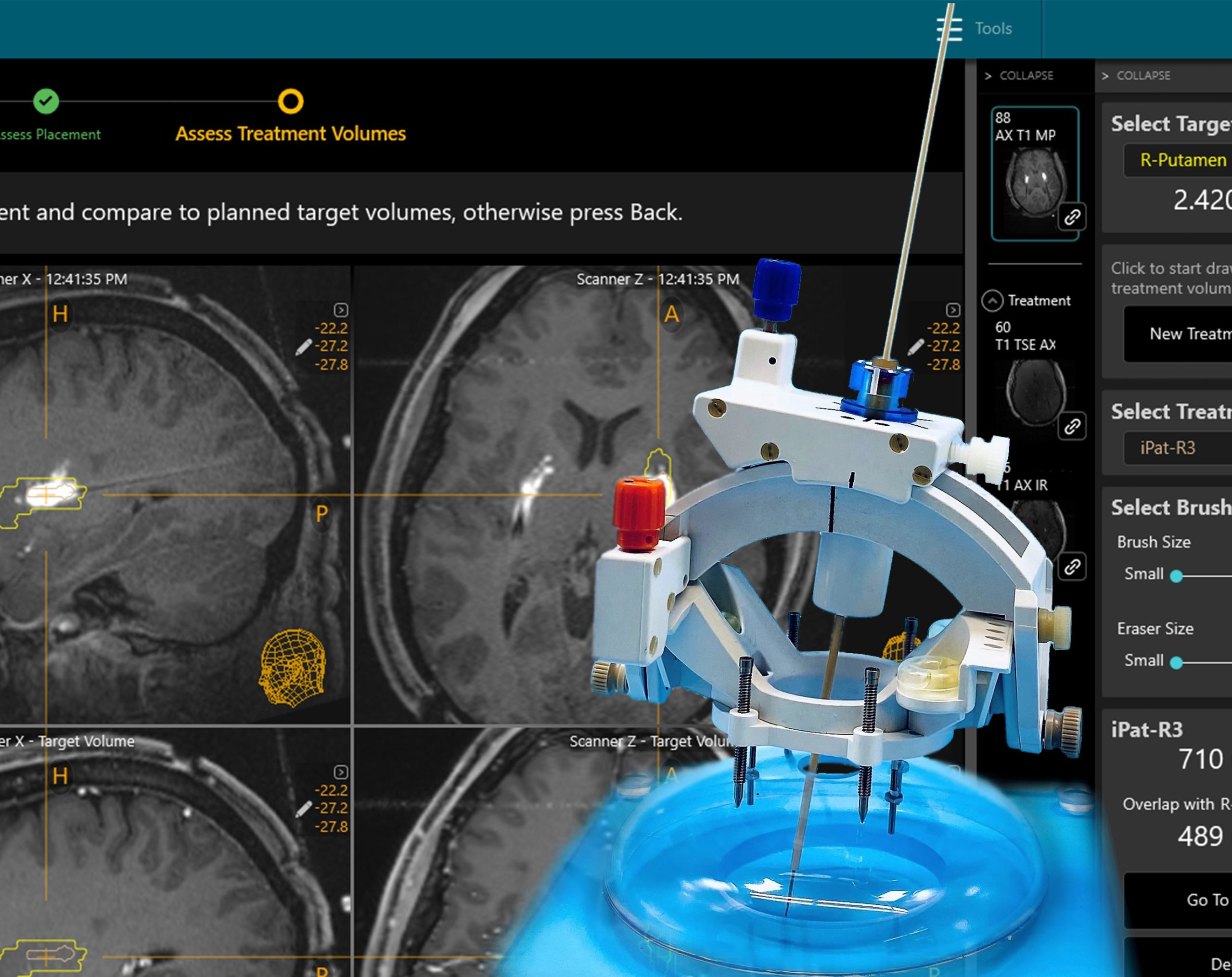
New Product: Next Generation SmartFrame Array™ Neuro Navigation System
We are pleased to introduce our first frame designed to empower OR-to-MR workflows - the SmartFrame Array™ Neuro Navigation System v.1.1. Learn how Array v.1.1 with pre-planning helps streamline Drug Delivery, Laser Catheter Insertion, and Biopsy procedures while enabling expanded workflow options. Array is compatible with Surgical Navigation System probes and trackers to facilitate OR-to-MR workflows.
ClearPoint Champions Patient Education Series - MRI-Guided DBS Journey
Hear from Arkansas-based husband and father, Richard Bowman about his MRI-Guided Deep Brain Stimulation (DBS) journey. After struggling with severe body pain that took over six years to diagnose, Richard shares his experience with Young Onset Parkinson’s and his journey with MRI-Guided DBS surgery.
Archived ClearPoint On-Demand Peer-to-Peer Webinar: Expanding Neurosurgical Techniques in the MRI Space
In case you missed last month's live broadcast, hear the recorded on-demand webinar where Dr. Hooman Azmi shares his institution's experience expanding neurosurgical techniques in the MRI space. Watch this latest edition to learn how to set up new MRI-guided neurosurgical sites. Click Here to Watch Now
Chapter One: The History of Interventional MRI
Chapter Two: Applications of Interventional MRI in Neurosurgery
Chapter Three: Institutional Experience with Establishing an iMRI Surgical Program
Meet Us in Paris July 11th - 13th at the 3rd Annual EU Gene Therapy for Neurological Disorders Conference
We will be exhibiting in-person at the 2022 Gene Therapy for Neurological Disorders European Conference in Paris, France. Stop by to discuss your delivery needs with our team, and attend sessions on ClearPoint-enabled preclinical studies and clinical trials.