From Joe Burnett, President & CEO:
This past quarter our team, together with our partners, has achieved four significant regulatory milestones that impact both our Functional Neurosurgery and Biologics and Drug Delivery businesses:
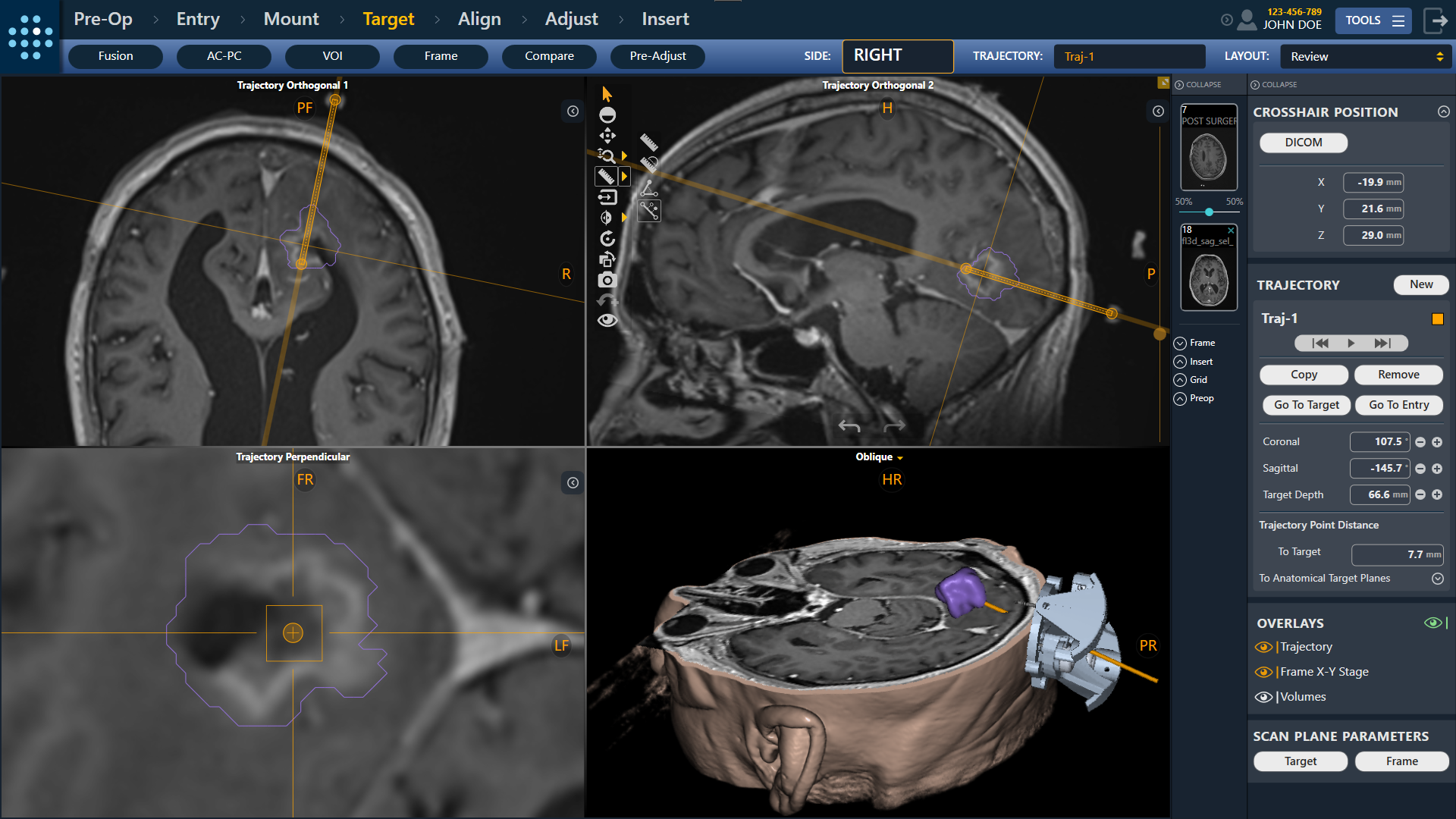
- FDA Clearance of the ClearPoint Prism™ Neuro Laser Therapy System - last Friday we announced that our partner, CLS , obtained 510(k) clearance for the laser, which we have exclusive rights to commercialize globally for neuro applications. Over the past decade, the ClearPoint team has supported approximately 2,000 laser ablation procedures in the United States and Europe. With the introduction of Prism, we believe Neurosurgeons will benefit from a streamlined workflow that does not require cooling due to the unique diffusing fiberglass tip of the Prism Laser Applicators. We also believe physicians and hospitals will benefit from a workflow that requires only One Room, One System, and One Team - the same ClearPoint team they have come to trust for neuro navigation support since 2010. If you plan to attend CNS in San Francisco, please book a time to demo Prism in our booth.
- 510(k) Clearance of the ClearPoint Maestro Brain Model - earlier this month we announced clearance of our Maestro anatomical segmentation analysis tool developed with our partner Philips. Maestro is currently in limited market release at select Neurosurgical and Neuroradiology centers and will be available for demonstration at our CNS booth.
- FDA Clearance of ClearPoint Neuro Navigation Software Version 2.1 - Also in September, our team received clearance for our next generation navigation software version 2.1. The 2.1 release builds on the tremendous success of our ClearPoint software to date, and includes many new features and improvements suggested by our neurosurgeon customers, while also setting the stage for the next generations of ClearPoint products. Our path forward will take full advantage of the capabilities of the Maestro Brain Model which will serve as the engine to drive further innovation in all of our products. You can also demo this at CNS.
- Marketing Authorization for PTC's Upstaza™ in Europe Delivered with SmartFlow Cannula: Finally, in July our partner PTC Therapeutics was granted marketing authorization by the European Commission for, Upstaza™, a gene therapy product for the treatment of AADC Deficiency. The labeling for Upstaza includes reference to our SmartFlow cannula explicitly in the surgical guide as the only infusion cannula to be used for delivery of this gene therapy. To say it another way, the very first marketed gene therapy ever approved for direct infusion into the brain will be administered exclusively with our device. We believe this first approval, along with the exhaustive bench, pre-clinical and clinical testing required for submission, is a sign of things to come for our more than 45 pharmaceutical partners that could see a similar path to approval. Our goal is for our SmartFlow family of cannula products to be referenced in the labeling for therapies across multiple partners and indications, ensuring that ClearPoint is the go-to delivery mechanism for neurosurgeons to deliver drugs to the brain and spine.
We are incredibly proud of the new innovations we bring to providers and partners and we are just getting started. As always, thank you for your continued support of our vision and our team. If you have any questions for me, please do not hesitate to reach out.
Announcing 510(k) Clearance for the ClearPoint Prism™ Neuro Laser Therapy System
ClearPoint Neuro has exclusive global rights to commercialize the CLS magnetic resonance (MR) guided laser interstitial thermal therapy (MRgLITT) system for neuro applications. The system comprises a clinical solution combining the ClearPoint navigation platform, the laser technology from CLS, and a thermal ablation monitoring software called Thermoguide™ from France-based medical device company Image Guided Therapy (IGT). Visit our website to learn more about the ClearPoint Prism™ Neuro Laser Therapy System.
510(k) Cleared - Not CE Marked - Currently in Limited Market Release in the U.S.
Announcing FDA Clearance for Software Version 2.1
510(k) Cleared - Not CE Marked
The main customer benefits of the 2.1 software include optimizing ease of use for clinicians, enhancing visualization of medical image datasets, providing a new set of trajectory planning tools, introducing new workflow tools for gene therapy clinical trials, and numerous performance and technical improvements which will help to streamline and optimize the clinical workflow. Schedule an in-booth demonstration at CNS in San Francisco:
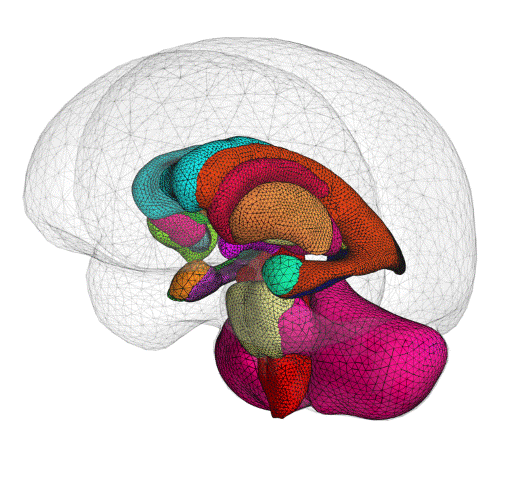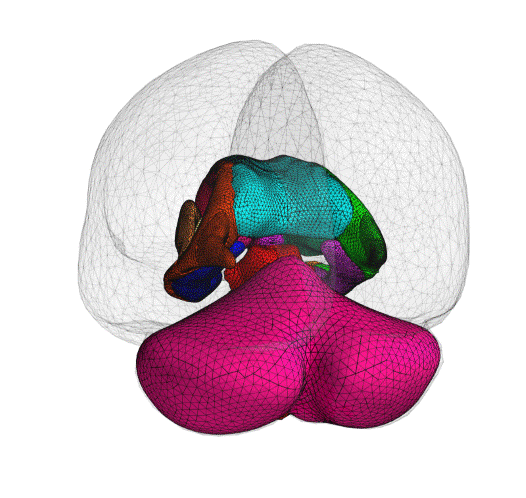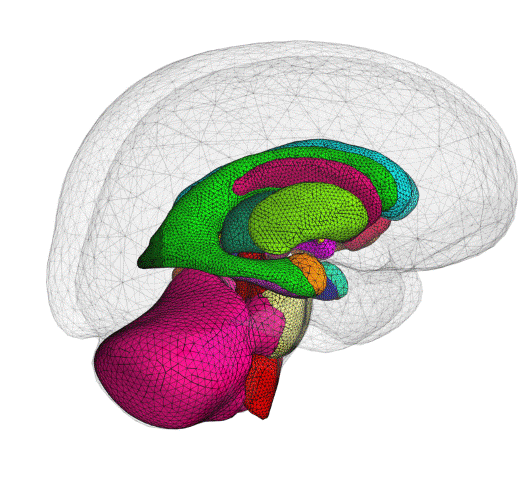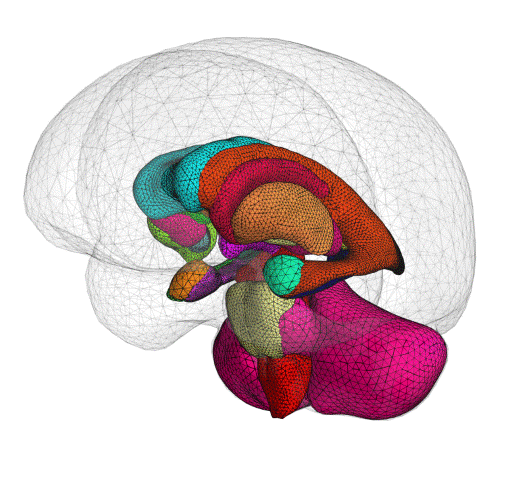
ClearPoint Maestro™ Brain Model now 510(k) Cleared
510(k) Cleared - Not CE Marked
We are pleased to announce that the ClearPoint Maestro™ Brain Model has received 510(k) clearance. Maestro is intended for automatic labeling, visualization, volumetric and shape quantification of segmentable brain structures from a set of MRI images. This software is intended to automate the process of identifying, labelling, and quantifying the volume and shape of brain structures visible in MRI images. Schedule an in-booth demonstration at CNS in San Francisco.
The ClearPoint Maestro™ Brain Model is an anatomical segmentation analysis tool that has been developed with our partner, Philips.
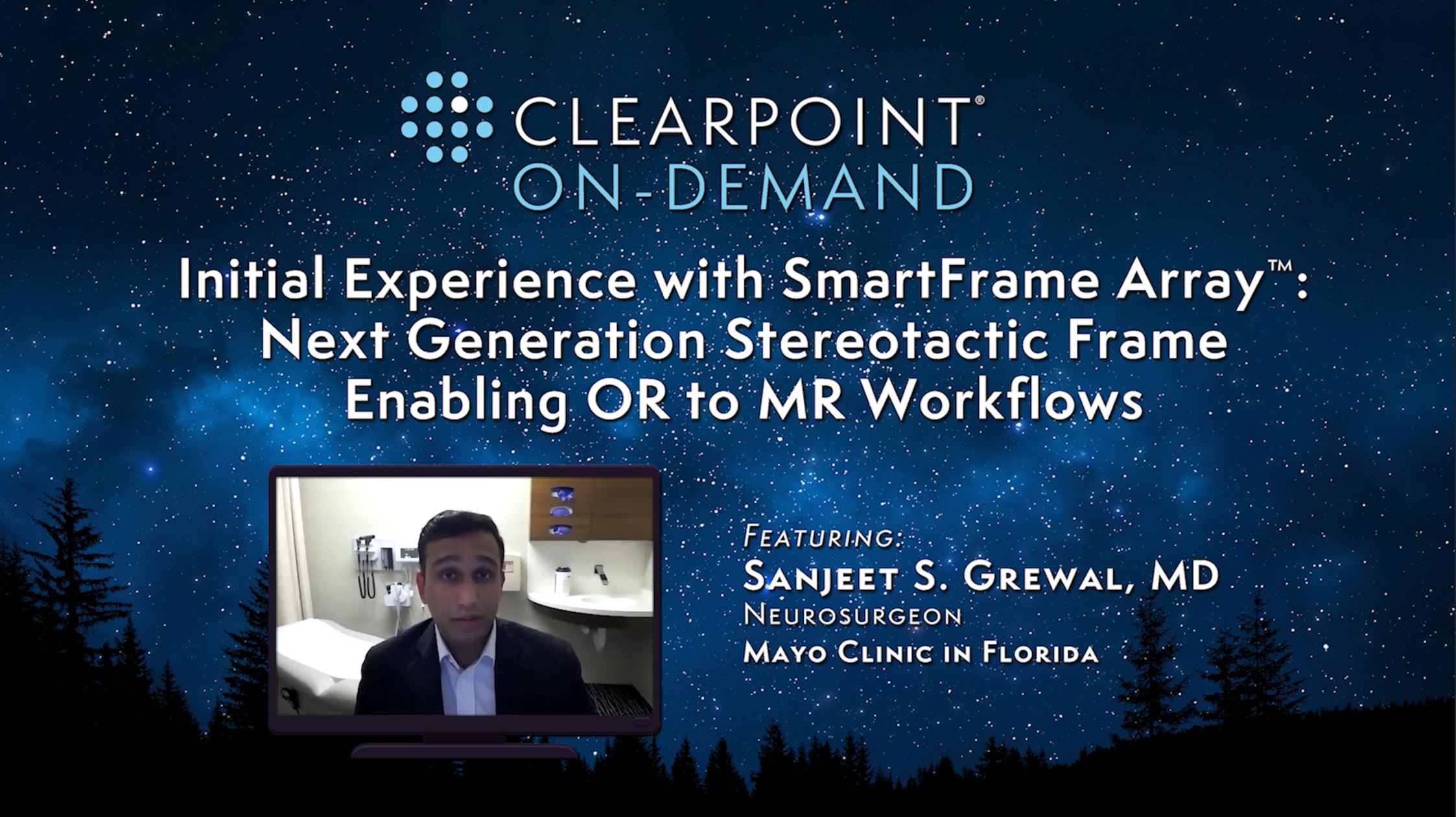
Watch ClearPoint On-Demand Peer-to-Peer Webinar: SmartFrame Array™, Next Generation Stereotactic Frame Enabling OR to MR Workflows
In case you missed it, please watch on-demand Dr. Sanjeet S. Grewal from the Mayo Clinic in Florida share his initial experiences with SmartFrame Array - the next generation stereotactic frame enabling OR to MR workflows.
ClearPoint Congratulates Partner PTC Therapeutics on Being Granted Marketing Authorization by the European Commission for Upstaza™: The First Disease-Modifying Treatment for AADC Deficiency
Upstaza™ is the first approved disease-modifying treatment for aromatic L-amino acid decarboxylase (AADC) deficiency, a rare and life-threatening disease impacting children, and is the first marketed gene therapy approved for direct infusion into the brain. The use of ClearPoint Neuro’s proprietary CE Marked SmartFlow® Neuro Cannula for minimally invasive infusion of the gene therapy is included in the Upstaza™ Summary of Product Characteristics (SmPC).
Join Us at One of Our Upcoming 2022 Patient & Family In-Person Outreach Events
At ClearPoint Neuro, we always put patients first and providing patients and their loved ones with education is one of our top priorities. ClearPoint sponsors in-person patient events across the United States. If you would like to participate, stay up-to-date on our upcoming Patient & Family In-Person Events.
Meet Us at Booth 248 in San Francisco on October 8th-12th at the 2022 CNS Annual Meeting: Neurosurgery Connected
ClearPoint Neuro will be exhibiting at the 2022 CNS Annual Meeting: Neurosurgery Connected San Francisco, California, where we will sponsor lecture SYM06B: Controversies in Movement Disorder Surgery on Saturday, October 8th at 1PM Pacific Time. During exhibit hall hours from Monday 10/10 - Wednesday 10/12, schedule time to demo our latest software innovations: ClearPoint Prism Neuro Laser Therapy System, ClearPoint software v.2.1 and the ClearPoint Maestro Brain Model in Booth 248.
Join Us in Rochester, Minnesota on October 13th-14th at the 2022 Mayo Clinic Stimulation Therapies for Epilepsy CME Course
We're excited to be exhibiting at the 2022 Mayo Clinic Stimulation Therapies for Epilepsy CME Course in Rochester, Minnesota on Thursday 10/13 & Friday 10/14 at the Hilton Rochester Mayo Clinic Area. Stop by the ClearPoint Neuro tabletop exhibit to meet members of our team and learn more about our portfolio of innovative neurosurgical technologies and educational resources