From Joe Burnett, President & CEO:
This has been an incredible quarter of many ClearPoint firsts. I am delighted to share that we have now treated the first clinical trial patient in a Glioblastoma study utilizing our ClearPoint Prism Neuro Laser Therapy System in Lund, Sweden. Introducing laser technology is our first foray into the therapy space. With our partner CLS' recent FDA clearance, the ClearPoint team looks forward to treating more clinical patients with Epilepsy and Brain Tumors in the coming years.
This quarter, we were also proud to support the first commercial gene therapy cases in Europe to treat AADC Deficiency together with our partner PTC Therapeutics. As we add more regulatory clearances for our products, we plan to support treatment of these very sick children in new geographies, beyond the U.S. and Europe.
Finally, we are pleased to announce that we have signed a lease for a beautiful 20,000 square foot (1858 square meter) manufacturing facility in Carlsbad, California, just up the street from our Solana Beach headquarters. Our new factory, combined with the over 30 new employees we added in 2022, will help prepare us for expanded future growth and provide continued support for our hospital and pharma partners. We will maintain our Irvine facility for the near-term, but look forward to welcoming our neurosurgeon customers and pharmaceutical partners to see first-hand how our products are designed and built.
Thank you for allowing the ClearPoint team to support you, your staff, and most importantly, your patients. Have a wonderful holiday. We'll see you in 2023!
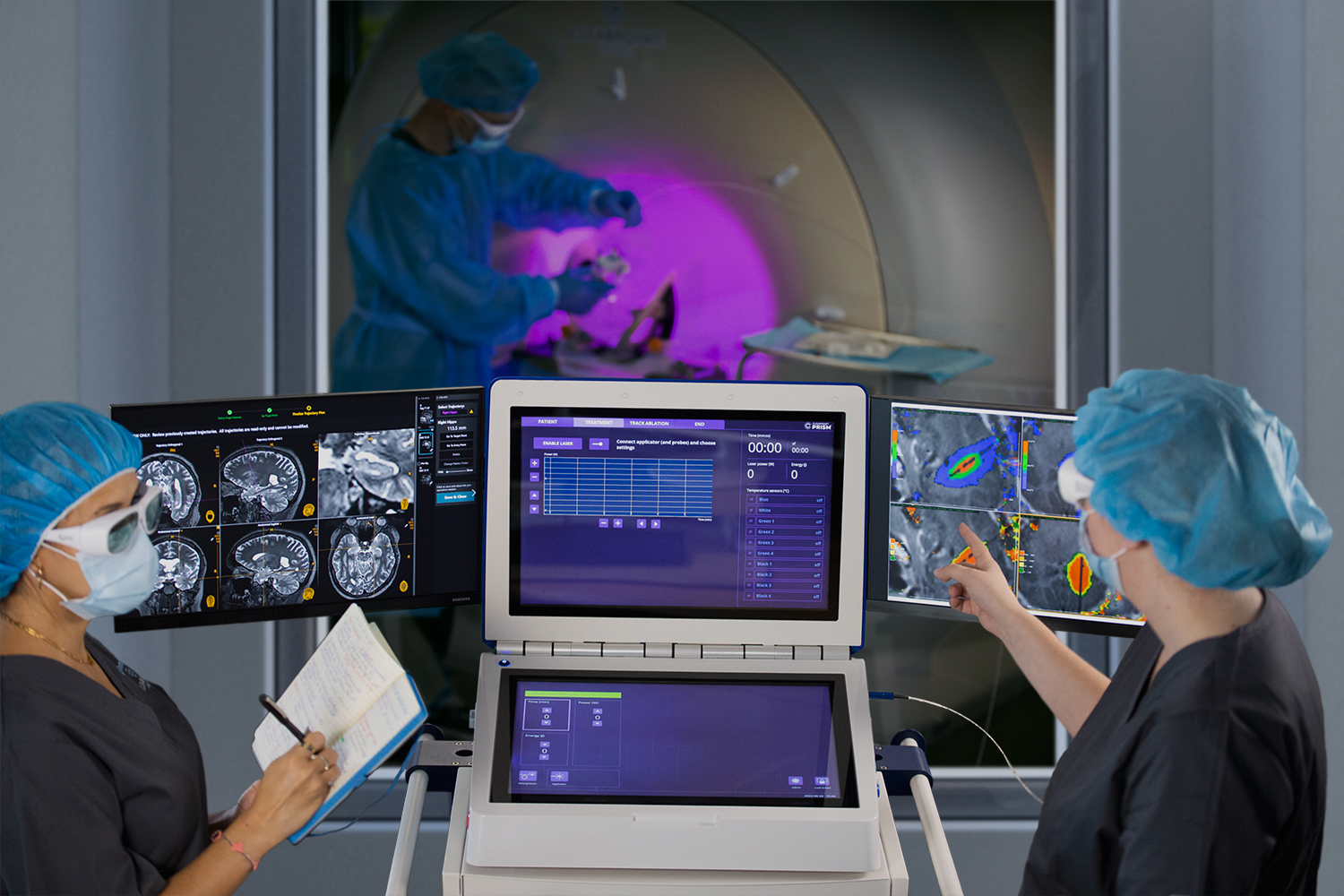
Announcing First Patient Enrolled in Glioblastoma Trial Using ClearPoint Prism™ Neuro Laser Therapy System at Skåne University Hospital
We’re excited to announce that the first patient has been enrolled in a Glioblastoma clinical trial using the ClearPoint Prism™ Neuro Laser Therapy System at Skåne University Hospital in Lund, Sweden. The Safety & Feasibility of MR-Guided Laser Thermal Ablation of Brain Lesions Study, sponsored by Clinical Laserthermia Systems AB (CLS), is a single-center, single-arm, prospective clinical trial evaluating the safety and feasibility of using the minimally invasive MR-guided laser therapy system to ablate tumors in up to five Glioblastoma patients.
Watch Our Latest ClearPoint On-Demand Peer-to-Peer Webinar: Data Driven Evolution of MRI-Guided Intra-Parenchymal Delivery for Gene Therapy Clinical Trials
In case you missed it, you can now access the latest edition of our ClearPoint On-Demand webinar series at your leisure. In this edition, Dr. Paul Larson, a leading gene therapy clinical trialist, discusses his experience with the data driven evolution of MRI-guided intra-parenchymal delivery for gene therapy clinical trials.
Featuring: Paul S. Larson, MD, FAANS
Chief of Neurosurgery at Southern Arizona VA & Professor of Neurosurgery at University of Arizona
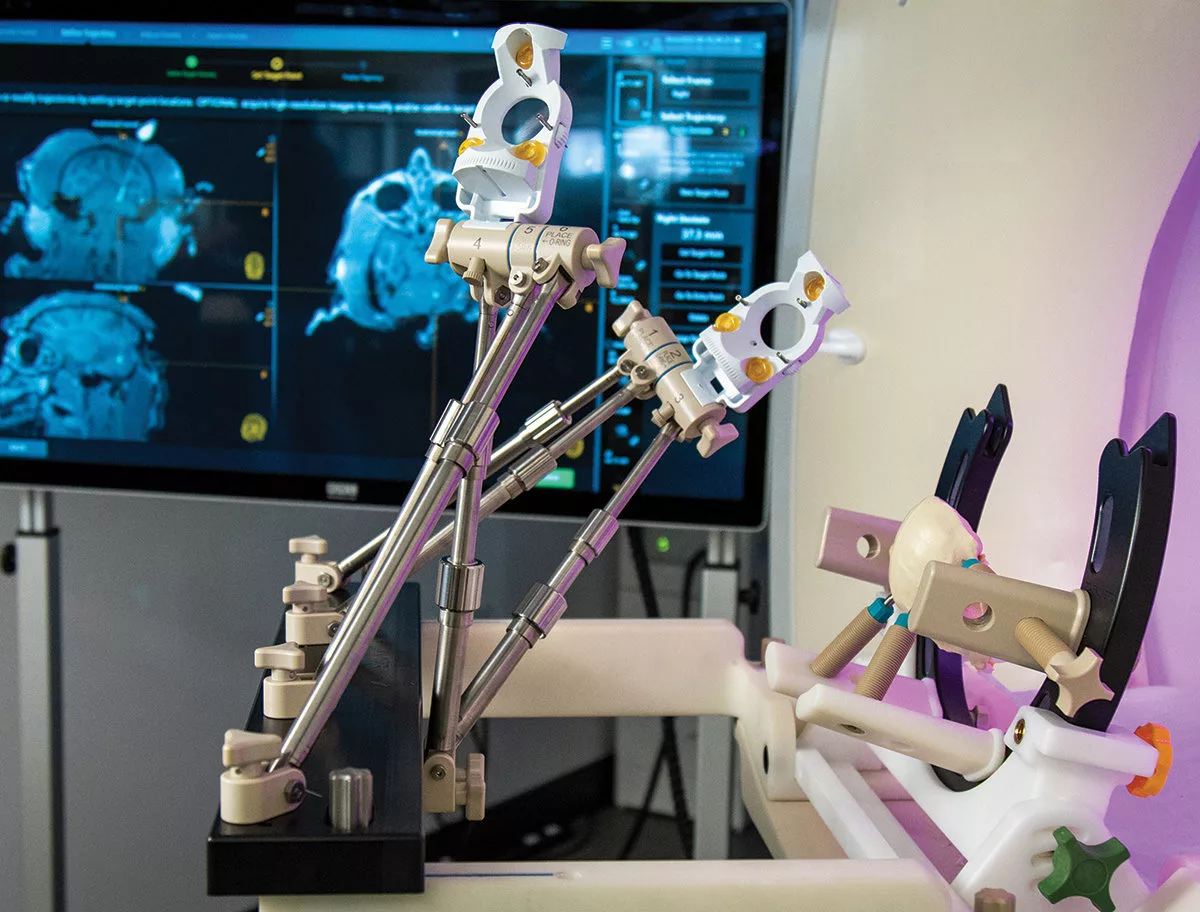
Introducing the ClearPoint Pre-Clinical Orchestra™ Frame
Providing versatile fixation & positioning options for your pre-clinical surgical needs
The ClearPoint Pre-Clinical Orchestra™ provides study directors with versatile options for head pinning and navigation frame mounting in various pre-clinical models. The unique design of the head fixation component enables prone, supine, or lateral positioning, with flexibility for MRI imaging coil placement. By mimicking the clinical head fixation frame and utilizing the clinical navigation platform, the Orchestra allows for a seamless transition from pre-clinical research and studies into the clinic.
Recent Peer-Reviewed Publications Featuring ClearPoint Neuro
Every quarter we add new Medline-indexed, peer-reviewed publications featuring use of ClearPoint technology across our portfolio of indications. Our bibliography currently features over 70 manuscripts, many of which are Open Access:
- Lee AT, et al. Targeting Accuracy and Clinical Outcomes of Awake versus Asleep Interventional Magnetic Resonance Imaging-Guided Deep Brain Stimulation for Parkinson's Disease: The University of California, San Francisco Experience. Neurosurgery. 91(5):p 717-725, November 2022.
- Keam, S.J. Eladocagene Exuparvovec: First Approval. Drugs. 82, 1427–1432 (2022). Published Online 14 September 2022.

Happy Holidays from Everyone Here at ClearPoint Neuro!
Throughout 2022, all of us at ClearPoint Neuro have been grateful for the opportunity to globally support our neurosurgeons, partners, and clinical researchers treat patients with debilitating neurological diseases/disorders to help improve quality of life for patients and their loved ones.