Convection Enhanced Delivery (CED)
Read more to learn about the Convection Enhanced Delivery (CED) technique and how it's utilized in clinical trials to deliver various gene therapies and therapeutic agents.
The ClearPoint Biologics Blog is an educational tool for healthcare providers, clinical researchers, and our partners to provide the reader with information on different key topics. If you have any questions regarding our biologics and drug delivery products or services, we’d love to hear from you:
Convection Enhanced Delivery (CED):
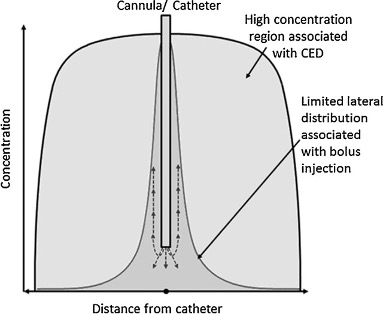
Convection enhanced delivery (CED) is a technique used for delivering therapeutics directly to the brain, bypassing the blood brain barrier, and has been found to enhance the distribution of small and large molecules within the interstitial space (Bobo et al., 1994). The CED method differs from that of standard diffusion in that it utilizes a pressure gradient at the cannula tip, which provides a much more uniform spreading of the infusate and also allows for larger infusate volumes to be delivered.
Image adapted from Lewis, O., Woolley, M., Johnson, D., Rosser, A., Barua, N. U., Bienemann, A. S., Gill, S. S., & Evans, S. (2016). Chronic, intermittent convection-enhanced delivery devices. Journal of neuroscience methods, 259, 47–56. https://doi.org/10.1016/j.jneumeth.2015.11.008 to demonstrate the difference between CED and standard diffusion.
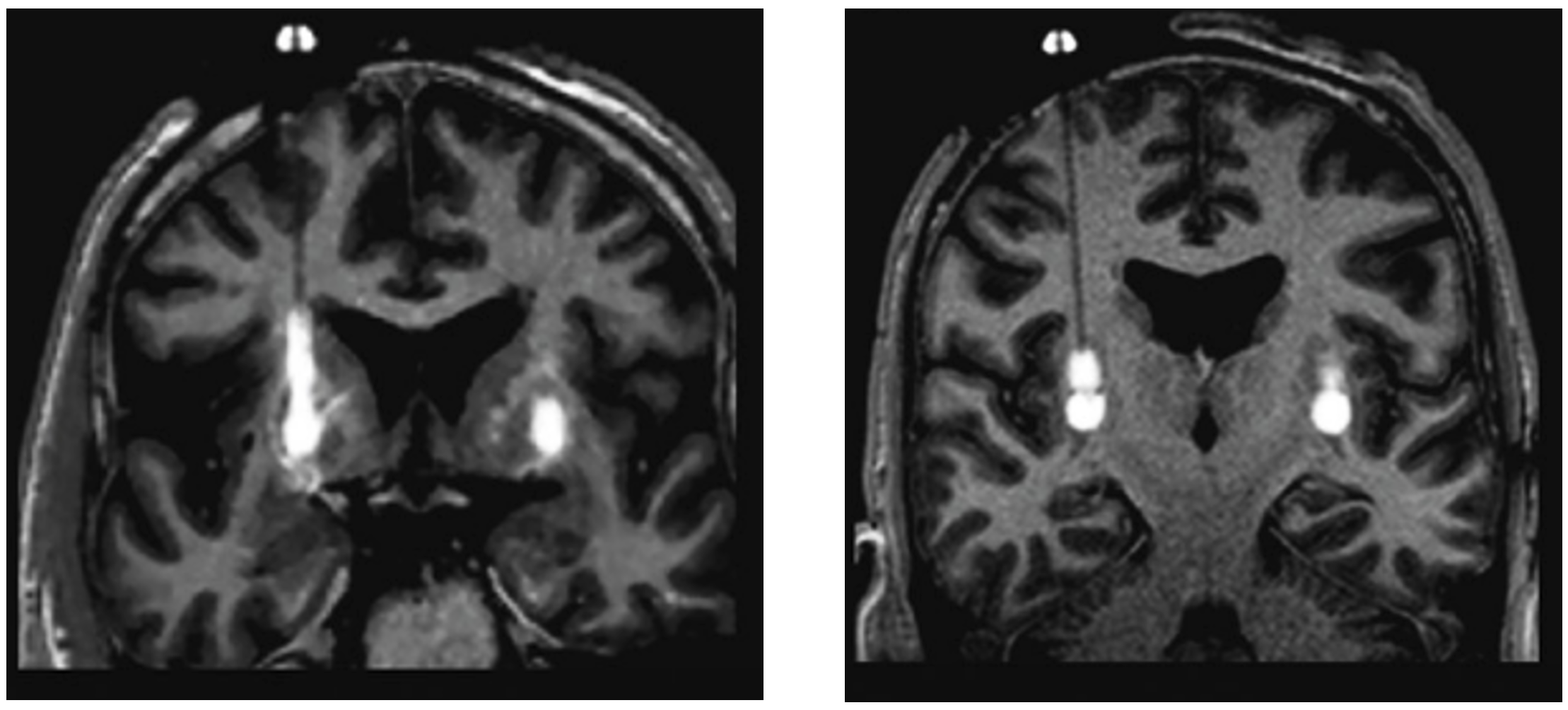
ClearPoint’s SmartFlow Neuro Ventricular Cannula has been designed specifically for delivering therapeutics directly to the brain for both preclinical studies as well as clinical trials. The stepped-tip construction prevents “reflux” or “back-flow” of the infusate as commonly seen with standard diffusion (Richardson et al., 2011; Han et al., 2016).
Image adapted from Richardson, R. M., Bankiewicz, K. S., Christine, C. W., Van Laar, A. D., Gross, R. E., Lonser, R., Factor, S. A., Kostyk, S. K., Kells, A. P., Ravina, B., & Larson, P. S. (2020). Data-driven evolution of neurosurgical gene therapy delivery in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry, 91(11), 1210–1218. https://doi.org/10.1136/jnnp-2020-322904 to demonstrate “reflux” or “back-flow” of infusate typically seen with standard diffusion (LEFT) as well as the uniform distribution from CED (RIGHT).
References:
- Bobo, R. H., Laske, D. W., Akbasak, A., Morrison, P. F., Dedrick, R. L., & Oldfield, E. H. (1994). Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences of the United States of America, 91(6), 2076–2080. https://doi.org/10.1073/pnas.91.6.2076
- Han SJ, Bankiewicz K, Butowski NA, Larson PS, Aghi MK. Interventional MRI-guided catheter placement and real time drug delivery to the central nervous system. Expert Review of Neurotherapeutics. 2016;16(6):635–639. doi:10.1080/14737175.2016.1175939
- Richardson MR, Kells AP, Martin AJ, Larson PS, et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotactic and Functional Neurosurgery. 2011;89(3):141–151. doi:10.1159/000323544
ClearPoint Neuro Biologics & Drug Delivery Solutions
Simple Solutions for Navigating a Complex Landscape
ClearPoint Neuro strives to support our partners through all stages of therapy development, leveraging our FDA-cleared and CE marked MRI-guided stereotactic system for neurological interventions. We offer solutions that will guide you from the benchtop to pre-clinical studies, through clinical trials, and into post-commercialization, with translational continuity. Together, we will navigate the regulatory landscape with an industry-leading cadence of innovation and caliber of services–all fully customizable to meet your project needs.
If you have any questions regarding our biologics and drug delivery products or services, we’d love to hear from you: