From Joe Burnett, President & CEO:
As we move into the last few months of the year, I'm delighted to share some highlights from this past quarter:
- ClearPoint Prism™ Neuro Laser Therapy System: We continue to execute on our limited market release and are in the process of collecting real-world experience for both epilepsy and brain tumors. To that end, the team from Skåne University Hospital presented data at ESSFN from their initial experience with our non-cooled laser to treat patients with recurrent glioblastoma from the podium.
- Biologics and Drug Delivery: Our strategy to build deeper and more strategic partnerships continues making progress resulting in a year-over-year growth of 55% in Q3. This growth is largely driven by our investments in tools and talent to add clinical, development, regulatory and other pre-clinical CRO services to our portfolio.
- New Product Introductions: Our received 510(k) clearance for our Array software version 1.2. This new software release is the second since we introduced the Array platform in 2021, demonstrating our ongoing commitment to rapid software iteration, and incorporation of meaningful voice of customer feedback.
- New Manufacturing Facility: We completed the transition to our brand new manufacturing facility in Carlsbad, and have permanently shut down our previous facility in Irvine, ahead of schedule.
Join Us for a Peer-to-Peer Webinar: ClearPoint Prism - Next Generation LiTT
Please join us on Thursday, January 25, 2024 at 12 PM Eastern (9 AM Pacific, 10 AM Mountain, & 11 AM Central) for this educational event as part of our ClearPoint On-Demand Webinar Series.
Dr. Mark Richardson, Director of Functional Neurosurgery at Massachusetts General Hospital and Associate Professor of Neurosciences at Harvard Medical School, will discuss his initial experience with the ClearPoint Prism™ Neuro Laser Therapy System, the next generation of laser interstitial thermal therapy.
Live Q&A available at the program's end.
Announcing FDA Clearance for Array Software Version 1.2
510(k) Cleared - Not CE Marked
We are pleased to announce 510(k) clearance and full market release of Array Software version 1.2.
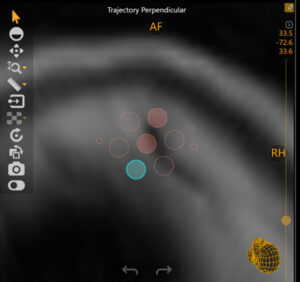
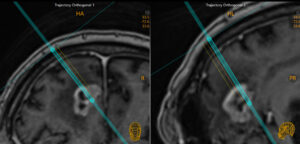
ClearPoint Array® software version 1.2 is designed to streamline MR-guided trajectory workflows from pre-planning through device insertion. Plan and visualize parallel trajectories with a virtual array tool for improved laser therapy, drug catheter placement and biopsy procedures from a single mounting position.
Array is compatible with Surgical Navigation System probes and trackers to facilitate OR-to-MR workflows.
Prism Laser Abstract ESSFN Podium Presentation
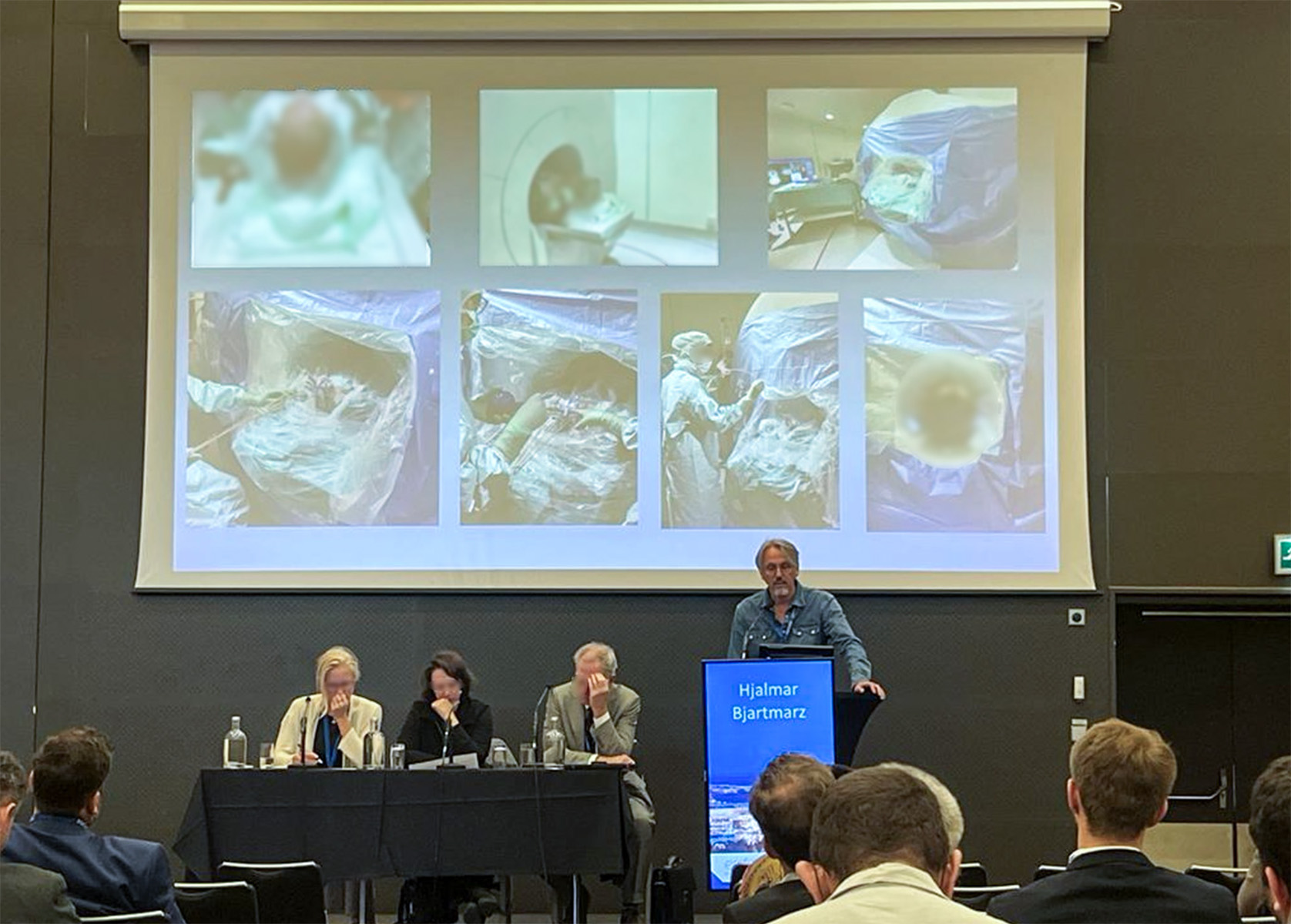
On September 28, Dr. Hjálmar Bjartmarz and team from of Skåne University Hospital presented an abstract entitled: Laser interstitial therapy using an uncooled laser catheter in a diagnostic MR suite on the podium at the XXVth Congress of the European Society for Stereotactic and Functional Neurosurgery (ESSFN) in Stockholm.
The abstract from the open-label, single center, early feasibility and safety phase 1-2 clinical trial featured results from 5 patients with recurrent glioblastoma. The team found that LITT, using a non-cooled laser catheter inside an outpatient MRI suite, was feasible and reproducible. They also concluded that the procedure may reduce risks associated with LITT procedures.
Initial Clinical Experience with Intraputaminal Delivery of Upstaza using ClearPoint Platform Presented at ESGCT
Dr. Luca D'Angelo and team from Sapienza Università di Roma presented a poster at the European Society of Gene and Cell Therapy (ESGCT) held in October in Brussels.
The poster entitled: Our initial experience with intraputaminal delivery of Upstaza using MRI-guidance for cannula placement and confirmation of infusion target coverage is the first peer-reviewed abstract documenting the clinical use of an approved, commercially-available gene therapy delivered through the SmartFlow cannula and guided by the ClearPoint navigation platform.

6349 Paseo Del Lago, Carlsbad, CA 92011
Stay Tuned for News on the Grand Opening in 2024
New Manufacturing & R&D Facility in Carlsbad, California
ClearPoint Neuro has completed the transition to our new manufacturing facility in Carlsbad, California ahead of schedule. This facility is now producing and shipping sellable product to customers.
The upgraded facility is ~20,000 sq. ft. and includes a 1,500 sq. ft. Class 8 Clean Room and a 1,200 sq.ft. R&D lab.
Designed to leverage best in-class lean manufacturing, warehouse, and shipping expertise.
Implementing the ClearPoint System within Mobile MRI Units
We're excited to offer a new educational video on how to setup the ClearPoint system for pre-clinical studies in a mobile MRI trailer.
Reach out to our Biologics team if you’d like access to the instructional video!
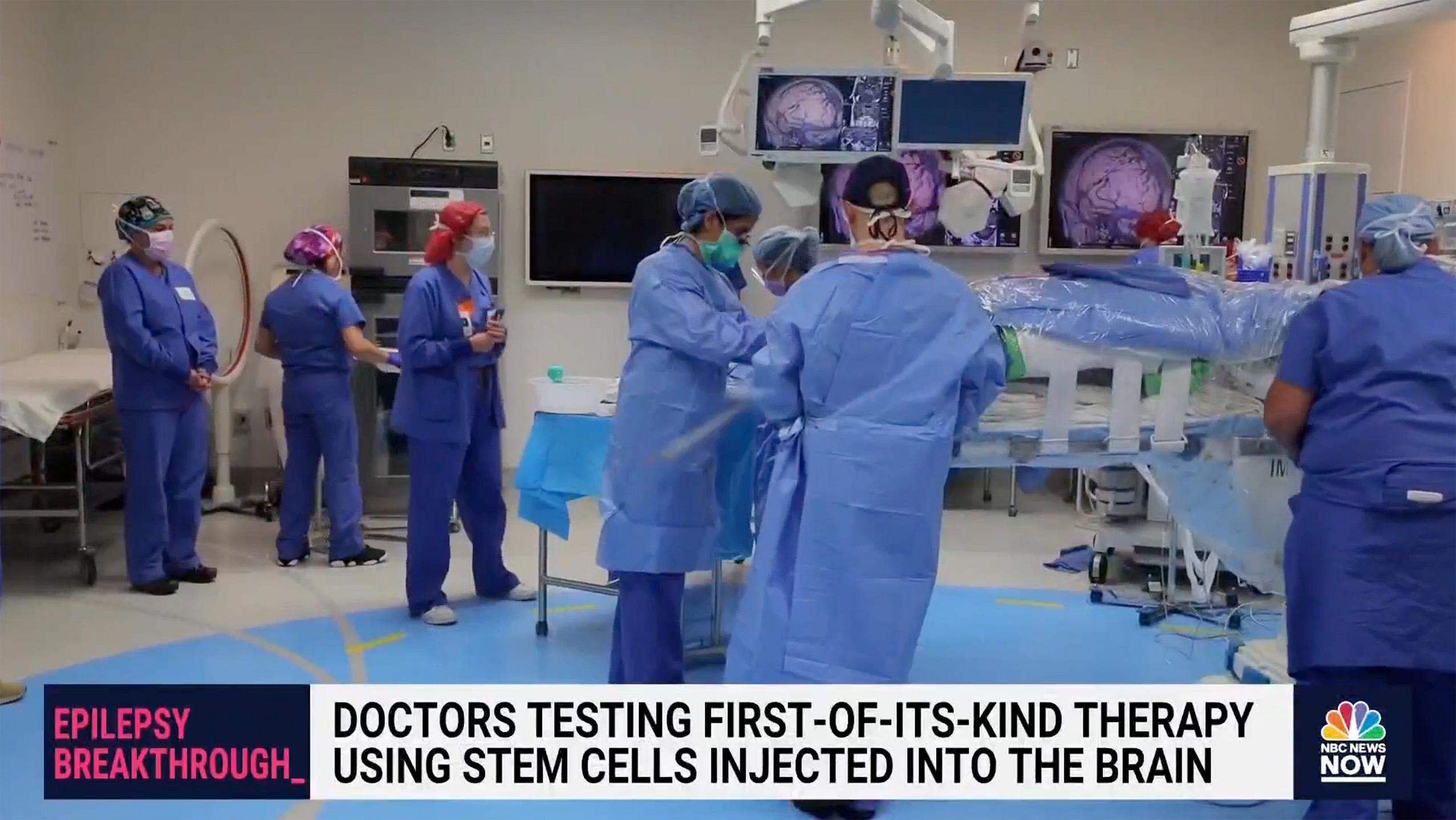
Doctors Test Experimental Cell Therapy to Combat Seizures in Epilepsy Patients
Congratulations to Dr. Sharona Ben-Haim and the entire neurosurgery team at UC San Diego on successfully delivering Neurona Therapeutics' groundbreaking investigational regenerative cell therapy for Drug Resistant Mesial Temporal Lobe Epilepsy.
The ClearPoint team is proud to have supported the procedure with MRI-guided navigation and the SmartFlow Cannula.
Recent Peer-Reviewed Publications Featuring ClearPoint Neuro
Pino IP, Darrow DP, Chen CC. MRI-aided SmartFlow convection delivery of DNX-2401: a pilot, prospective case series. World Neurosurgery, 2023, ISSN 1878-8750.1
1. The ClearPoint system enables intraprocedural MRI guidance for a number of neurological therapies and approved clinical trials.