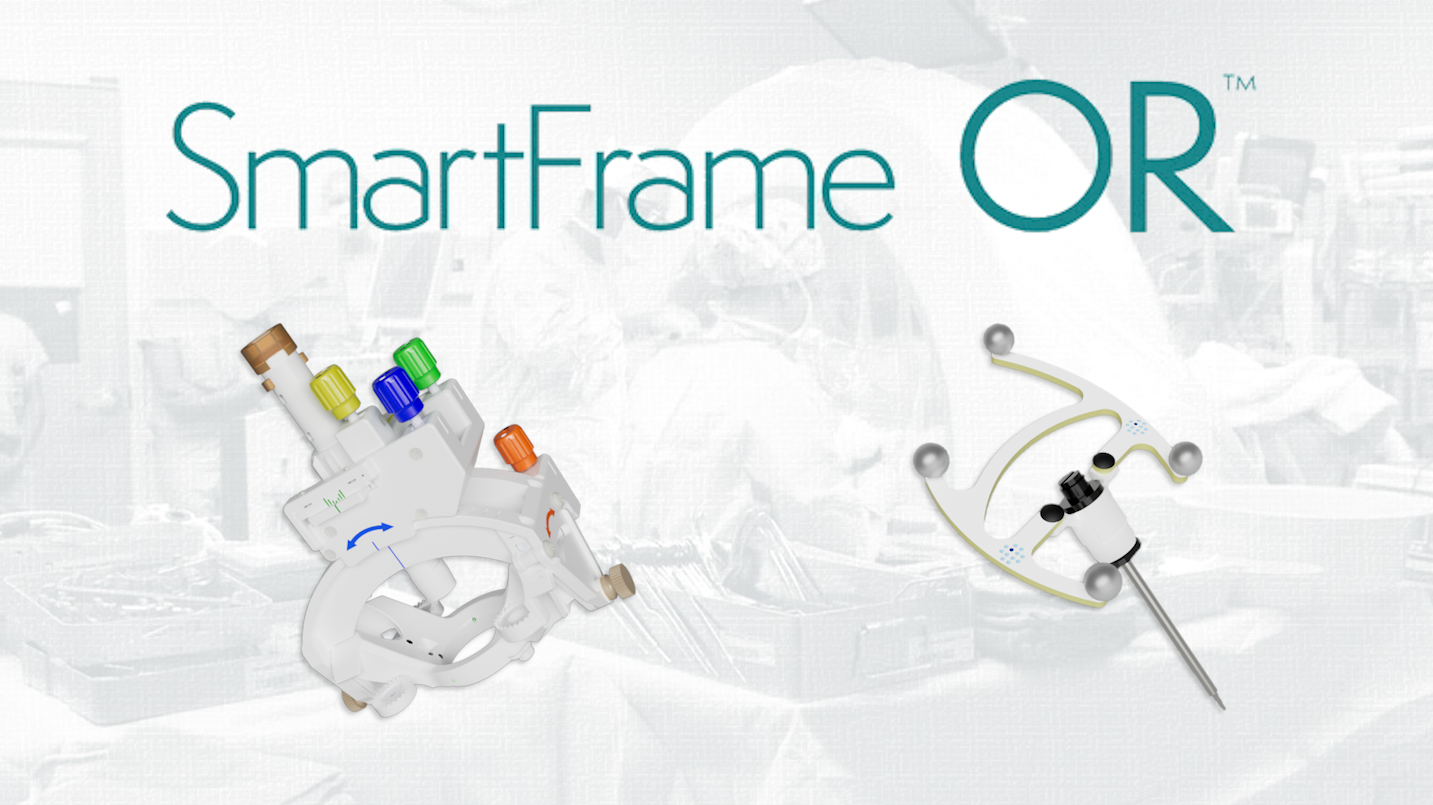
Join Our Next Webinar: Initial DBS Experience with the SmartFrame OR™ Platform
Dr. Andrew Conner, Assistant Professor of Neurosurgery and Neurosurgeon at the University of Oklahoma Health Sciences Center, will be sharing his initial DBS experience with the SmartFrame OR Platform - ClearPoint's first solely Operating Room-based product.
Join us on Wednesday, September 18, 2024 at 12:00 PM Pacific / 3:00 PM Eastern for this educational webinar hosted by one of your peers, with live Q&A at the end of the presentation.
ClearPoint Neuro Announces Full Market Release of ClearPoint Prism® 3T Laser Therapy System and SmartFrame OR™ Platform
Read the Full Press Release Here
The ClearPoint Prism® Neuro Laser Therapy System:
Precision. Unparalleled.
The ClearPoint Prism Neuro Laser Therapy System can enable thermometry precision with accurate damage assessments1 and superb resolution through a simple set-up which, by design, does not require extra cooling.
The SmartFrame OR™ Platform:
Delivery. Dialed-In.
The SmartFrame OR Platform, ClearPoint's first solely Operating Room-based product, enables performance of intraprocedural adjustments and workflows that help reduce registration errors.
"Deep Brain Stimulation (DBS) has been proven to be a safe and effective strategy to manage patients with medically intractable Parkinson's Disease, Essential Tremor, and Dystonia. In its more than 30-year history in the US, this highly effective therapy has been mostly performed in highly specialized neurosurgical centers, which, because of their relative rarity, has resulted in only about 5% of eligible patients being treated. The development of the SmartFrame OR is anticipated to be a significant step towards accomplishing the triple aim of achieving excellent outcomes in an accessible and appropriate clinical setting, with broad patient acceptance, because it is expected to allow DBS procedures to be performed in a much larger spectrum of hospitals, by a larger contingent of well-trained neurosurgeons."
Dr. Kim Burchiel
Professor of Neurological Surgery
The Oregon Health & Science University
*Data on file at ClearPoint Neuro
Watch our Latest ClearPoint On-Demand Webinar: ClearPoint Prism® - Next Generation LiTT
In case you missed it, you can now stream the latest edition of our educational peer-to-peer ClearPoint On-Demand webinar series at your leisure.
Dr. Mark Richardson, Director of Functional Neurosurgery at Massachusetts General Hospital and Associate Professor of Neurosciences at Harvard Medical School, shares his initial experience with the ClearPoint Prism Neuro Laser Therapy System, the next generation of laser interstitial thermal therapy.
Take a closer look at ClearPoint Neuro while at CNS in Houston. We can't wait to show you all that is new.
Join ClearPoint at the 2024 CNS Annual Meeting in Houston: September 28 - October 02
We're honored to both exhibit and support three educational symposia for Stereotactic/Functional Neurosurgeons and Neurosurgical Oncologists at CNS 2024:
- SYM06A: Epilepsy Surgery Symposium - Integrating Practical Approaches for All Ages
- SYM06B: Movement Disorder Surgery - Novel Approaches and Measuring the Impact of Chronic Readings
- SYM17A: Brain Tumor Update - Management of Malignant Brain Tumors
Register for one of these symposia, or stop by Booth #1741 to see how we're taking our experience beyond the MRI into the Operating Room with the SmartFrame OR Platform, and into Therapy with the ClearPoint Prism Neuro Laser Therapy System, and supporting the future of Neurosurgery through Biologics and Drug Delivery trials.
Meet Us in Rome at the 2024 ESGCT Annual Meeting
ClearPoint Neuro is proud to be a sponsor at the 2024 European Society of Gene & Cell Therapy Annual Meeting in Rome, Italy from October 22nd - 25th.
Visit us at booth C12 to see how ClearPoint is dedicated to providing services and solutions for all phases of the drug development pipeline – from early preclinical studies, through clinical trials and commercialization.
Taiwan Food and Drug Administration Approves SmartFlow Cannula
We are pleased to announce the regulatory approval of the SmartFlow® Cannula in Taiwan by the Taiwan Food and Drug Administration (TFDA).
This marks ClearPoint's first product approval in Asia, with more submissions planned in the future.
Recent Partner Updates
In Chronological Order
- July 11, 2024: AskBio receives FDA Fast Track and MHRA Innovation Passport designations for AB-1005 investigational GDNF gene therapy for Parkinson’s disease
- July 1, 2024: ASPIRE-FTD Phase 1/2 Clinical Trial Opens in the U.S. at The Ohio State University Wexner Medical Center [AviadoBio]
- June 20, 2024: Aspen Neuroscience Announces Use of the ClearPoint Navigation System for All Enrolled Patients in ASPIRO Clinical Trial
- June 18, 2024: Neurona Therapeutics Receives FDA’s Regenerative Medicine Advanced Therapy (RMAT) Designation for NRTX-1001 in Focal Epilepsy
- June 3, 2024: uniQure Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for Investigational Gene Therapy AMT-130 in Huntington’s Disease
- May 30, 2024: BlueRock Therapeutics receives FDA Regenerative Medicine Advanced Therapy designation for Parkinson’s disease cell therapy candidate bemdaneprocel